Figure 7.
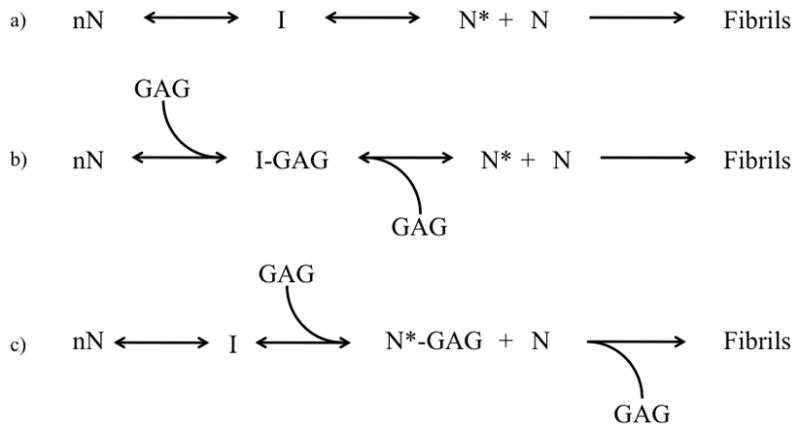
Proposed model of glycosaminoglycan effect on AL fibril formation
Under normal conditions, shown in (a), an unknown number of natively folded AL-103 monomers (nN) undergo a conformational change. This altered conformation is prone to aggregate, forming an intermediate I en route to the formation of a nucleus N*. Additional monomers interact with N* to form amyloid fibrils. AL-09 and AL-103 differ in the efficiency of the first two steps; AL-09 appears to populate the nucleus in a very favorable way without the enhancement of cofactors such as GAGs. Based on our fibril formation and calorimetry data, we hypothesize that glycosaminoglycans (GAGs) accelerate AL-103 fibril formation by interacting with and stabilizing a prefibrillar conformation. This is depicted in (b) and (c). In (b), the interaction occurs between the GAG molecule and an intermediate, while in (c) the interaction occurs with the nucleus itself. At this point our data do not give any indication as to which of (b) or (c) are more likely.
