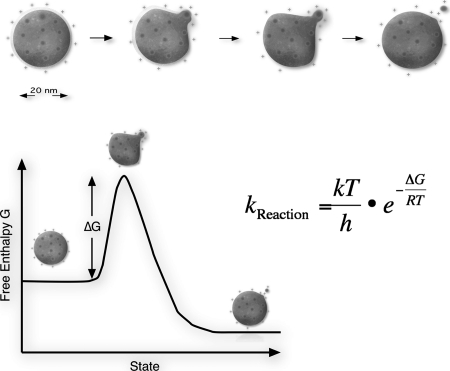
Fig. 1.
The ion evaporation process. An individual ion leaves the charged droplet in a solvated state. The electric field strength at the surface of a droplet is so high that the energy required to increase the droplet surface is rapidly compensated by the gain because of Coulombic repulsion. kReaction, reaction rate constant; k, Boltzmann constant; T, temperature; h, Planck's constant; R, ideal gas constant.