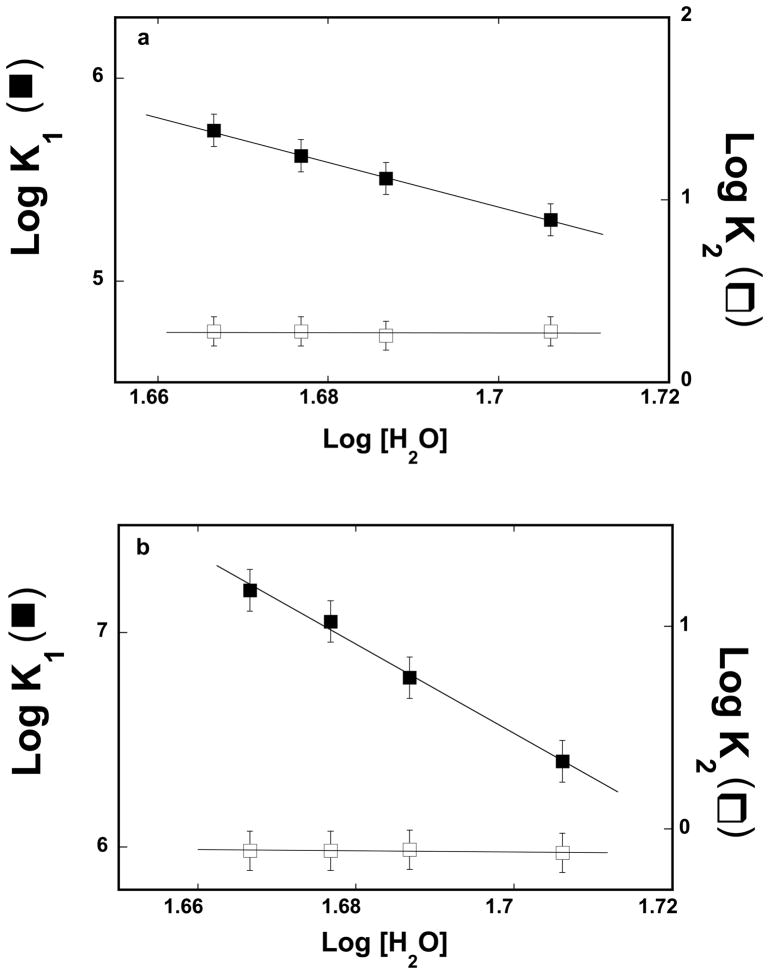
Figure 5.
a. The dependence of the logarithm of the partial equilibrium constants, K1 (■) and K2 (❑) upon the logarithm of the water concentration for binding of the ssDNA 10-mer, dεA(pεA)9, to the strong DNA-binding subsite of the ASFV pol X, as defined by the two-step kinetic mechanism (eq, 7), in buffer C (pH 7.0, 10°C) containing different concentrations of glycerol. The solid lines are the linear least-squares fits of the experimental plots, which provide the slopes ∂LogK1/∂Log[H2O] = −11.1 ± 3.0 and ∂LogK2/∂Log[H2O] = −0.10 ± 0.20. b. The dependence of the logarithm of the partial equilibrium constants, K1 (■) and K2 (❑), upon the logarithm of the water concentration for the binding of the ssDNA 20-mer, dεA(pεA)19, to the total DNA-binding site of the ASFV pol X, as defined by the two-step kinetic mechanism (eq, 7), in buffer C (pH 7.0, 10°C) containing different concentration of glycerol. The solid lines are the linear least-squares fits of the experimental plots, which provide the slopes ∂LogK1/∂Log[H2O] = −20.7 ± 6.0 and ∂LogK2/∂Log[H2O] = −0.2 ± 0.20 (details in text).