Figure 3.
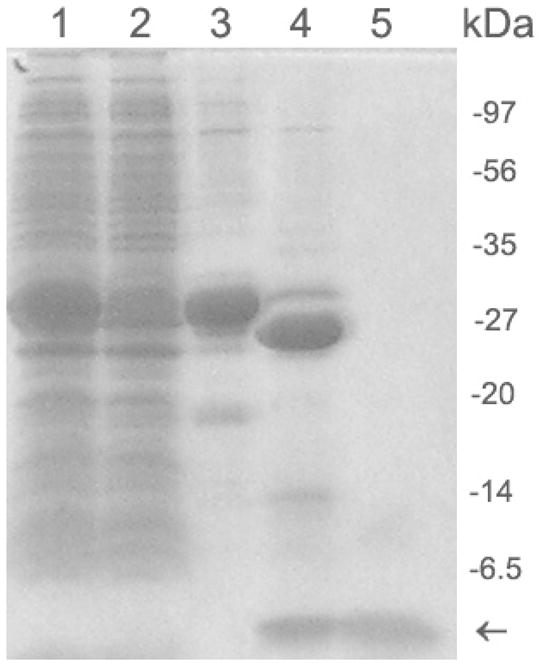
15% SDS-PAGE analysis of cells exacts and cleavage of GST-Aβ(1–40) fusion protein. Lane 1, soluble supernatant from cell treated with 0.5% (w/v) sodium lauroyl sarcosinate and 0.8% Triton X-100 (v/v). Lane 2, soluble supernatant treated with sodium lauroyl sarcosinate and TX-100, and flowed through glutathione agarose column. Lane 3, GST-Aβ(1–40) fusion protein eluted from glutathione agarose. Lane 4: a cleavage mixture after incubation of GST-Aβ(1–40) fusion protein with Factor Xa for 16 h. Lane 5: the cleaved protein solution was filtered through an Amicon Ultra-15 centrifugal filter unit (30-kDa-MWCO). The arrow indicates the position of Aβ(1–40) having a molecular mass of 4–6 kDa. The cleavage of the fusion protein was performed by 1U of Factor Xa per 20 μg of the fusion protein at 16°C and pH 9.0. The molecular mass of GST tag is ~26 kDa.
