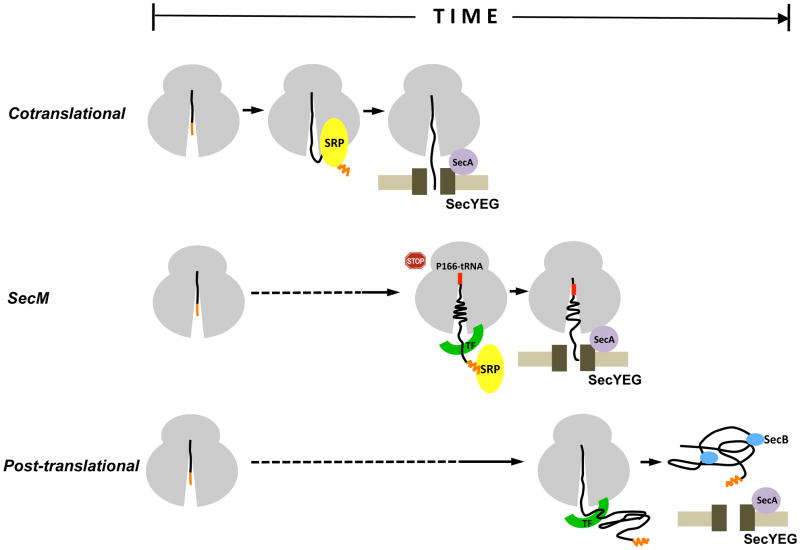
Fig. 8.
The SecM signal peptide functions as a molecular timer. In conventional cotranslational targeting (top), SRP binds to the signal peptide as soon as it emerges from a translating ribosome and rapidly guides RNCs to the SecYEG complex. In post-translational targeting (bottom), a presecretory protein is first completely synthesized and released from ribosomes. The protein is then targeted to the SecYEG complex by molecular chaperones such as SecB, which keep it in a translocation-competent conformation. In SecM targeting (middle), the SecM signal peptide specifies an intermediate rate of targeting that ensures that RNCs reach the IM immediately after synthesis of the arrest motif (residues 150–166).