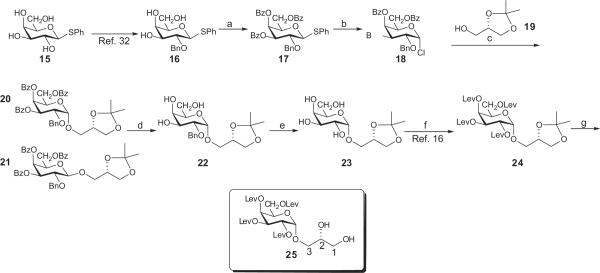
Scheme 1.
Reagents and conditions: (a) 4.9 equiv BzCl, CH2Cl2,C6H5N, DMAP (cat), rt, 24 h, 91%; (b) Cl2 in CCl4 (excess), CH2Cl2,0 °C, 30 min, hex-1-ene (excess), 69%); (c) 1.4 equiv 19, 2,4,6-tri-tert-butylpyrimidine (22.5 g, 184 mmol), 4 Å molecular sieves, AgOTf (15 g, 58.2 mmol), −40 °C, 15 min, 61%); (d) NaOMe (excess), CH2Cl2, MeOH, Dowex 50WX8, CH2N2, 91%); (e) H2, Pd/C, 2,4,6-tri-tert-butylpyrimidine, EtOAc, 15 min, 87%; (f) levulinic acid (6 equiv), DCC (7 equiv), 4-dimethylaminopyridine (cat), EtOAc, 94%); (g) AcOH, MeOH, reflux, 5 min, 83%.