Figure 3.
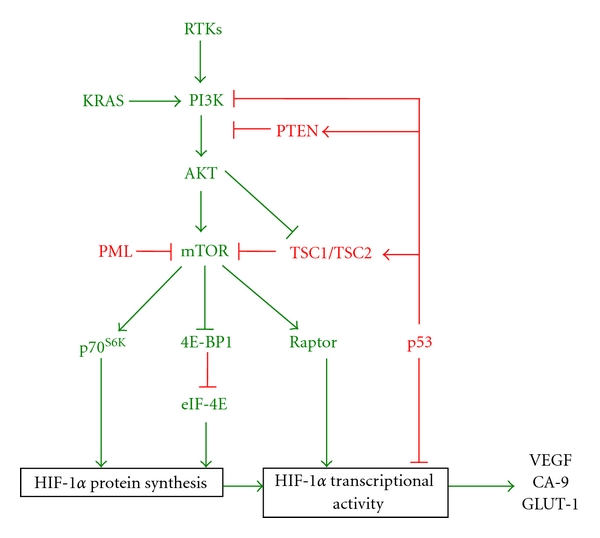
Regulation of HIF-1α by the PI3K pathway. Stimulation of receptor tyrosine kinases (RTKs) by growth factors or activating mutations in the RAS family of GTPases, such as KRAS, results in activation of PI3K. Activated PI3K enables the phosphorylation and activation of AKT. AKT in turn activates mammalian mTOR by disrupting the inhibitory interaction of the TSC1/TSC2 complex with mTOR. mTOR enhances HIF-1 signalling in a multimechanistic way (a) By activating the translational machinery proteins p70S6K and EIF-4E leading to enhanced protein synthesis of HIF-1α and (b) By promoting the interaction of HIF-1α with the coactivator p300, possibly by direct phosphorylation of HIF-1α via interaction with the scaffold protein raptor. Additionally, deletion or loss of function of negative regulators of the PI3K pathway leads to enhanced signalling and activation of HIF-1. These include PTEN, p53, PML, and TSC1/2. The phosphatase PTEN dephosphorylates the products of PI3K, inhibiting activation of AKT and subsequent downstream targets. p53 inhibits PI3K signalling at multiple levels; p53 inhibits transcription of the catalytic subunit of PI3K (p110α), enhances transcription of PTEN and TSC2, interacts with HIF-1α, and recruits MDM2, targeting HIF-1α for proteosomal degradation. PML inhibits mTOR under hypoxia and TSC1/2 interacts with and prevents activation of mTOR in the absence of PI3K activation.
