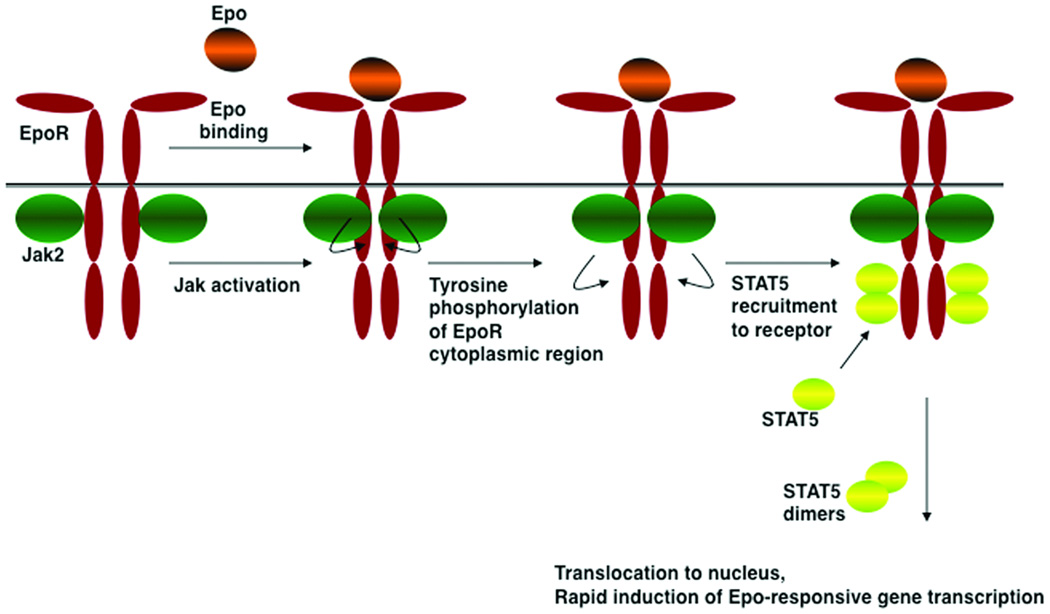
Figure 1. Model for EpoR activation mechanism.
The EpoR is thought to be expressed at the cell surface as a dimer in the absence of ligand. Epo binding proceeds by an asymmetrical association, first via the high affinity Epo site 1 followed by the lower affinity site 2 interaction (not shown), which stimulates a reorientation of EpoR monomers within the dimeric complex and subsequent activation of the receptor-associated Jak2 kinase, presumably by a transphosphorylation mechanism. Jak2 phosphorylates tyrosine residues in the EpoR cytoplasmic domain and itself, providing docking sites for molecules with phosphotyrosine binding motifs. STAT5 proteins are principal signaling molecules for EpoR, undergoing homodimerization after recruitment and phosphorylation by the receptor-Jak2 complex. STAT5 mediates erythroid survival, proliferation and differentiation signals. Additional signaling cascades are activated by the EpoR (not shown), however their role in erythropoiesis in vivo remains less understood relative to STAT5.