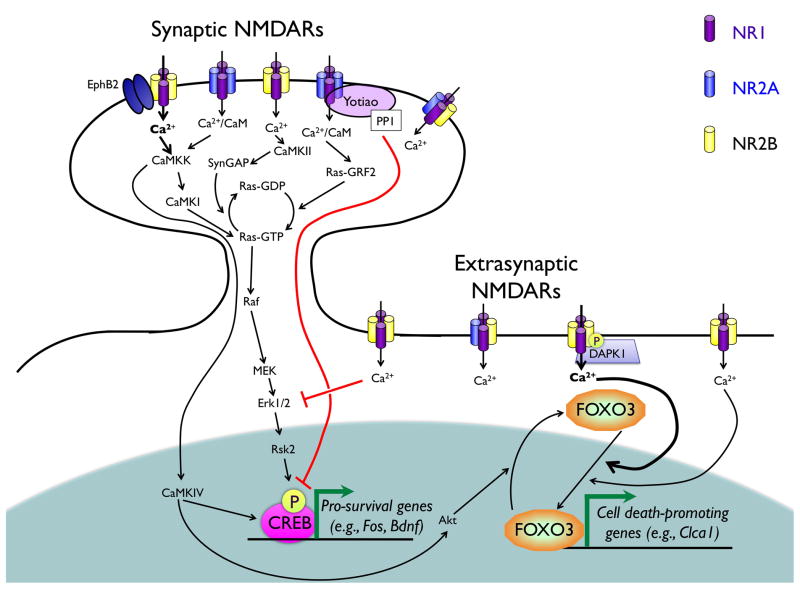
FIGURE 6. Different pools of NMDARs are capable of effecting distinct changes in gene transcription.
Synaptic NMDARs are capable of signaling through the CaMKs and the Ras/Raf/MEK/Erk/Rsk2 pathway to phosphorylate CREB and initiate transcription of a variety of pro-survival genes. Additionally, synaptic NMDAR activation of CaMKK and CaMKIV leads to the activation of Akt and subsequently the phosphorylation and nuclear export of the transcription factor FOXO3, thereby inhibiting transcription of various cell death-inducing genes. In contrast, activation of extrasynaptic NMDARs opposes the effects of synaptic NMDAR activation. Activation of extrasynaptic NMDARs inhibits the activation of Erk1/2, thereby reducing CREB phosphorylation. Additionally, extrasynaptic NMDARs induce the nuclear import of FOXO3 and the subsequent transcription of pro-death genes; this is potentiated by DAPK1. Subunit composition can also lend specificity to synapse-to-nucleus signaling by NMDARs. NR2A-containing NMDARs are capable of interacting with and selectively activating Ras-GRF2; activation of NR2B-containing NMDARs leads to the activation of CaMKII and the subsequent phosphorylation of SynGAP. While NR2B-containing NMDARs have also been shown to interact with Ras-GRF1 (not shown), how that signal is integrated with SynGAP activation remains to be determined. The EphB2 receptor has been shown to interact with the NR1 subunit and potentiate NMDAR-mediated calcium influx and downstream signaling cascades by phosphorylating the NR2B subunit. Lastly, the scaffolding protein Yotiao can bind to NR1 splice variants containing the C1 cassette and tether PKA and PP1 to the channel complex. While it is not known if this tethering is important for PKA-mediated NMDAR signaling (not shown), this interaction could mediate PP1-dependent signaling to the nucleus and dephosphorylation of CREB.