Abstract
Background:
Relatively little is known about the management and outcomes of Aboriginal children with renal failure in Canada. We evaluated differences in dialysis modality, time spent on dialysis, rates of kidney transplantation, and patient and allograft survival between Aboriginal children and non-Aboriginal children.
Methods:
For this population-based cohort study, we used data from a national pediatric end-stage renal disease database. Patients less than 18 years old who started renal replacement treatment (dialysis or kidney transplantation) in nine Canadian provinces (Quebec data were not available) and all three territories between 1992 and 2007 were followed until death, loss to follow-up or end of the study period. We compared initial modality of dialysis and time to first kidney transplant between Aboriginal children, white children and children of other ethnicity. We examined the association between ethnicity and likelihood of kidney transplantation using adjusted Cox proportional hazard models for Aboriginal and white children (data for the children of other ethnicity did not meet the assumptions of proportional hazards).
Results:
Among 843 pediatric patients included in the study, 104 (12.3%) were Aboriginal, 521 (61.8%) were white, and 218 (25.9%) were from other ethnic minorities. Hemodialysis was the initial modality of dialysis for 48.0% of the Aboriginal patients, 42.7% of the white patients and 62.6% of those of other ethnicity (p < 0.001). The time from start of dialysis to first kidney transplant was longer among the Aboriginal children (median 1.75 years, interquartile range 0.69–2.81) than among the children in the other two groups (p < 0.001). After adjustment for confounders, Aboriginal children were less likely than white children to receive a transplant from a living donor (hazard ratio [HR] 0.36, 95% confidence interval [CI] 0.21–0.61) or a transplant from any donor (HR 0.54, 95% CI 0.40–0.74) during the study period.
Interpretation:
The time from start of dialysis to first kidney transplant was longer among Aboriginal children than among white children. Further evaluation is needed to determine barriers to transplantation among Aboriginal children.
Compared with non-Aboriginal people, Aboriginal adults with end-stage renal disease in Canada have lower rates of kidney transplantation, the optimal treatment for renal failure.1–4 Most studies to date that have examined health outcomes among Canadian Aboriginal people with kidney disease have focused on adults.1–8 Relatively little is known about the outcomes among Aboriginal children with renal failure. A single-centre cohort study from the province of British Columbia reported that Aboriginal children who received a kidney transplant had similar short-term, but poorer long-term allograft survival than white children.9 No further studies have examined differences in modality of renal replacement treatment or the likelihood of kidney transplantation among Aboriginal children with renal failure.
We performed an observational cohort study of children beginning renal replacement treatment in Canada. We compared differences in dialysis modality, time spent on dialysis, rates of kidney transplantation, and graft and patient survival between Aboriginal children, white children and children of other ethnicities.
Methods
Study design and data source
We obtained ethical approval of the study design from the University of Calgary Conjoint Health Research Ethics Board and funding from the Alberta Children’s Hospital Foundation. Using the Canadian Pediatric End-stage Renal Disease Database, we established a cohort of all patients less than 18 years old in nine Canadian provinces (patients in the province of Quebec were not included because their data were not released to investigators) and all three territories who started renal replacement treatment between Jan. 1, 1992, and Dec. 31, 2007. We excluded recipients of multiple organ transplants (i.e., a kidney and another organ). Patients were followed from the start of the renal replacement treatment until death, loss to follow-up or end of the study period.
The Canadian Pediatric End-stage Renal Disease Database is a novel database that was created by linking data from the Canadian Organ Replacement Register with data from the Canadian Institute for Health Information (CIHI) Discharge Abstract Database.10 The Canadian Organ Replacement Register data have been assessed for quality.11,12 When medical chart data were compared with register data, agreement exceeded 97% for baseline demographic characteristics and was 71% for type of renal disease.11 Hazard ratios (HRs) for death were similar whether calculated using register data or chart data.12 The register data captured 98.5% of renal transplants performed in Canada when compared with data from the CIHI Discharge Abstract Database.11
Exposure variables and potential confounders
Ethnicity, as determined by the health care professional responsible for reporting to the Canadian Organ Replacement Register, was classified for our study as white, Aboriginal (First Nations, Inuit or Métis, including Aboriginal people living on federal reserves) or other (black, Asian, Indian subcontinent, Mid-East/Arabian, Pacific Islander, other or unknown).
We used median neighbourhood income (classified by quintile) as a measure of socioeconomic status. These income data were compiled by linking 2001 Statistics Canada census data with postal codes for the patients’ residences.
We used distance from the patient’s residence at the start of treatment to the nearest pediatric renal care centre as a measure of geographic remoteness of residence. The geographic coordinates of residences and renal care centres were determined from postal codes and entered into geographic information systems software (ArcGIS 3.0, Esri, Calif.) to determine shortest distances by road between the patient’s residence and the closest pediatric renal care centre. The distances were categorized as follows: < 150 km, 150–300 km and > 300 km.
The primary cause of renal failure was categorized as congenital, genetic, glomerulonephritis or autoimmune disease, or other or unknown. We also examined differences in prevalence of blood groups by ethnic group.
Outcome measures
We evaluated the following outcomes: initial renal replacement modality (hemodialysis, peritoneal dialysis or pre-emptive transplant), time on dialysis, time to first kidney transplant (classified by source [any donor, living donor and deceased donor]), graft survival and overall survival.
Statistical analysis
Demographic and baseline clinical characteristics were described with medians and interquartile ranges (IQRs) or with proportions, as appropriate. A significance level of α ≤ 0.05 was used for all statistical tests. We compared characteristics by ethnic group using χ2 tests for categorical variables. We adjusted three-way comparison p values within each categorical variable using the Bonferroni correction for multiple comparisons. All analyses were performed with R software (The R Project for Statistical Computing, www.r-project.org).
We compared initial modality of dialysis between the three study groups. We also compared median time spent on dialysis (from start of dialysis to first kidney transplant, censored at death) between the three groups using the Kruskal–Wallis rank-sum test. Differences in unadjusted time to transplantation and graft survival between the three groups were assessed with the Kaplan–Meier analysis and log-rank test.
We used Cox proportional hazard models to examine the association between ethnicity and likelihood of kidney transplantation within the study period from any donor source and then, separately, from living donors and deceased donors. To satisfy assumptions for proportional hazards, the models for transplants from living and deceased donors were stratified by age at start of renal replacement treatment and adjusted for sex, socioeconomic status, cause of renal failure and distance to nearest pediatric renal care centre. The model for transplant from any source was stratified by age at start of renal replacement treatment and by cause of renal failure and was adjusted for sex, socioeconomic status and distance to nearest pediatric renal care centre. Stratification allowed different baseline hazards for each category of primary renal disease and age, as appropriate. The “other” ethnic group was removed from the Cox models, because this category did not meet proportional hazards assumptions. Adjusted models therefore compare data for Aboriginal and white children only. Proportional hazards assumptions were tested based on the scaled Schoenfeld residuals.13 Interaction terms were used to determine whether the association between ethnicity and likelihood of kidney transplantation varied by distance to the nearest pediatric renal care centre and socioeconomic status.
We assessed differences in overall unadjusted survival by ethnic group using the Kaplan–Meier analysis and log-rank test.
We dealt with missing data using multiple imputation methods. More Aboriginal children had missing demographic information on socioeconomic status and residence location (22.1% v. 8.3% of white children). Therefore, we used multiple imputation methods by chained equations in R.14 Missing values were imputed by means of generating plausible values based on other variables in the data (sex, ethnicity, age at start of renal replacement treatment, initial renal replacement modality, time spent on dialysis, and other variables) using polytomous regression (categorical variables with more than two categories). Complete data were analyzed using Cox proportional hazards models. This analysis was repeated five times, and estimates from each analysis were averaged. We compared hazard ratios for likelihood of kidney transplantation with and without multiple imputation and found no differences in results or conclusions. Therefore, we present the results without imputation.
Results
A total of 858 pediatric patients began renal replacement treatment during the study period. Fifteen patients received multiple organs and were excluded from the analysis. Of the remaining 843 patients included in the study, 104 (12.3%) were Aboriginal, 521 (61.8%) were white children and 218 (25.9%) were from other ethnic minorities.
The total follow-up time was 5991 person-years, with a median follow-up of 6.83 (IQR 3.00–10.62) years. The median follow-up was 8.00 years (IQR 3.91–11.25) among white children, 6.04 (IQR 2.73–9.65) years among Aboriginal children and 5.54 (IQR 2.10–9.00) years among children of other ethnicity.
The demographic and clinical characteristics of the study cohort are outlined in Table 1. Congenital renal disease was the primary cause of end-stage renal disease in 18.3% of the Aboriginal children, 33.2% of the white children and 22.9% of the children of other ethnicity. The median age at the start of renal replacement treatment did not differ significantly between the three groups (overall median age 13.0 [IQR 7.7–15.6] years; p = 0.50). A greater proportion of Aboriginal than white children lived more than 300 km from the nearest pediatric renal care centre (48.1% v. 27.6%) and were in the lowest neighbourhood income quintile (43.3% v. 14.4%). Proportions of ABO blood groups in each of the three ethnic groups are shown in Table 1. The most prevalent blood groups among the Aboriginal and white children were A and O.
Table 1:
Demographic and clinical characteristics of children who started renal replacement treatment between Jan. 1, 1992, and Dec. 31, 2007, by ethnic group
| Characteristic | Ethnic group; no. (%) of patients | p value† | ||
|---|---|---|---|---|
| Aboriginal n = 104 |
White n = 521 |
Other* n = 218 |
||
| Age at start of renal replacement treatment, yr | ||||
| < 1 | 8 (7.7) | 41 (7.9) | 14 (6.4) | 1.00 |
| 1–5 | 8 (7.7) | 47 (9.0) | 29 (13.3) | 0.74 |
| 5–10 | 16 (15.4) | 91 (17.5) | 41 (18.8) | 1.00 |
| 10–15 | 37 (35.6) | 179 (34.4) | 65 (29.8) | 1.00 |
| 15–18 | 35 (33.7) | 163 (31.3) | 69 (31.7) | 1.00 |
| Sex | ||||
| Female | 65 (62.5) | 249 (47.8) | 87 (39.9) | 0.001 |
| Male | 39 (37.5) | 272 (52.2) | 131 (60.1) | |
| Primary cause of renal disease | ||||
| Congenital | 19 (18.3) | 173 (33.2) | 50 (22.9) | 0.003 |
| Genetic | 11 (10.6) | 74 (14.2) | 27 (12.4) | 1.00 |
| Glomerulonephritis or autoimmune disease | 44 (42.3) | 140 (26.9) | 60 (27.5) | 0.02 |
| Other or unknown | 30 (28.8) | 134 (25.7) | 81 (37.2) | 0.03 |
| Blood type | ||||
| A | 30 (28.8) | 181 (34.7) | 55 (25.2) | 0.16 |
| AB | –‡ | 20 (3.8) | 5 (2.3) | 1.00 |
| B | –‡ | 41 (7.9) | 28 (12.8) | 0.006 |
| O | 45 (43.3) | 209 (40.1) | 74 (33.9) | 0.91 |
| Unknown | 27 (26.0) | 70 (13.4) | 56 (25.7) | < 0.001 |
| Neighbourhood income, quintile | ||||
| 1 (lowest) | 45 (43.3) | 75 (14.4) | 53 (24.3) | < 0.001 |
| 2 | 14 (13.5) | 93 (17.9) | 55 (25.2) | 0.11 |
| 3 | 11 (10.6) | 84 (16.1) | 39 (17.9) | 1.00 |
| 4 | 8 (7.7) | 117 (22.5) | 25 (11.5) | < 0.001 |
| 5 (highest) | 6 (5.8) | 114 (21.9) | 30 (13.8) | < 0.001 |
| Unknown | 20 (19.2) | 38 (7.3) | 16 (7.3) | 0.002 |
| Distance from pediatric renal care centre, km | ||||
| < 150 | 25 (24.0) | 267 (51.2) | 148 (67.9) | < 0.001 |
| 150–300 | 16 (15.4) | 83 (15.9) | 16 (7.3) | 0.03 |
| > 300 | 50 (48.1) | 144 (27.6) | 37 (17.0) | < 0.001 |
| Unknown | 13 (12.5) | 27 (5.2) | 17 (7.8) | 0.08 |
Includes black, Asian, Indian subcontinent, Mid-east/Arabian, Pacific Islander, other or unknown.
χ2 test. Three-way comparison p values were adjusted using the Bonferroni correction for multiple comparisons.
Cell sizes less than five are not reported owing to privacy rules of the Canadian Institute for Health Information.
Dialysis modality
Among children who started dialysis as the first form of renal replacement treatment, hemodialysis was the initial modality for 48.0% (47/98) of the Aboriginal children, 42.7% (178/417) of the white children and 62.6% (102/163) of the children of other ethnicity (p < 0.001). The time from start of dialysis to first kidney transplant was longer among the Aboriginal children (median 1.75 [IQR 0.69–2.81] years) than among the white children (median 0.75 [IQR 0.08–1.75] years) and children of other ethnicity (median 0.67 [IQR 0.00–1.75] years) (p < 0.001). Table 2 shows the proportions by ethnic group of children who never received dialysis or a kidney transplant and those who spent less than one year, one to two years or more than two years on dialysis.
Table 2:
Time from start of dialysis to first kidney transplant, by ethnic group
| Time to first transplant, yr | Ethnic group; no. (%) of patients | p value† | ||
|---|---|---|---|---|
| Aboriginal n = 104 |
White n = 521 |
Other* n = 218 |
||
| 0‡ | 6 (5.8) | 104 (20.0) | 55 (25.2) | 0.001 |
| < 1 | 16 (15.4) | 155 (29.8) | 47 (21.6) | 0.01 |
| 1–2 | 19 (18.3) | 103 (19.8) | 30 (13.8) | 0.76 |
| > 2 | 29 (27.9) | 90 (17.3) | 37 (17.0) | 0.16 |
| No transplant during study period | 34 (32.7) | 69 (13.2) | 49 (22.5) | < 0.001 |
Includes black, Asian, Indian subcontinent, Mid-east/Arabian, Pacific Islander, other or unknown.
χ2 test. Three-way comparison p values were adjusted using the Bonferroni correction for multiple comparisons.
No dialysis (pre-emptive transplant).
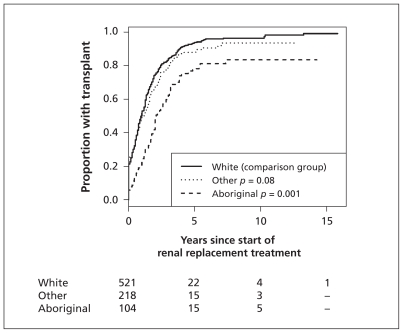
Time to transplantation
During the study period, 70 Aboriginal children (67.3%), 452 white children (86.8%) and 169 children of other ethnicity (77.5%) underwent transplantation. Figure 1 shows the unadjusted time to transplantation by ethnic group. After adjustment for confounders, Aboriginal children were 46% less likely to receive a transplant from any source than were white children with the same time elapsed since start of dialysis (adjusted HR 0.54, 95% confidence interval [CI] 0.40–0.74). In addition, fewer Aboriginal children had pre-emptive transplants (6 [5.8%] Aboriginal, 104 [20.0%] white, 55 [25.2%] other; p = 0.001).
Figure 1:
Kaplan–Meier analysis of time to first kidney transplantation among all children who received transplants, by ethnic group.
The proportion of children who received their first transplant from a living donor was 32.9% (n = 23) of Aboriginal children, 57.1% (n = 258) of white children and 42.6% (n = 72) of children of other ethicity (p < 0.001). Compared with white children who had the same time elapsed since start of dialysis, Aboriginal children were 64% less likely to receive a transplant from a living donor (adjusted HR 0.36, 95% CI 0.21–0.61) and 38% less likely to receive a transplant from a deceased donor (adjusted HR 0.62, 95% CI 0.42–0.92) (Table 3).
Table 3:
Factors associated with relative likelihood of kidney transplantation, by source of transplant*
| Variable | Source of transplant; adjusted HR† (95% CI) | ||
|---|---|---|---|
| Any source | Living donor | Deceased donor | |
| Ethnic group‡ | |||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Aboriginal | 0.54 (0.40–0.74) | 0.36 (0.21–0.61) | 0.62 (0.42–0.92) |
| Sex | |||
| Female | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Male | 1.11 (0.91–1.35) | 1.12 (0.86–1.47) | 1.05(0.78–1.41) |
| Primary cause of renal disease | |||
| Congenital or genetic | –§ | 1.00 (ref) | 1.00 (ref) |
| Glomerulonephritis or autoimmune disease | 0.43 (0.31–0.60) | 0.60 (0.43–0.85) | |
| Other or unknown | 0.55 (0.40–0.76) | 0.58 (0.40–0.83) | |
| Income quintile | |||
| 1 (lowest) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2 | 1.26 (0.92–1.72) | 1.30 (0.83–2.03) | 1.15 (0.76–1.75) |
| 3 | 1.03 (0.75–1.43) | 0.80 (0.51–1.25) | 0.82 (0.52–1.29) |
| 4 | 1.12 (0.83–1.52) | 1.02 (0.69–1.55) | 0.84 (0.53–1.32) |
| 5 (highest) | 1.30 (0.96–1.76) | 1.20 (0.80–1.80) | 1.02 (0.65–1.60) |
| Distance from pediatric renal care centre, km | |||
| < 150 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 150–300 | 0.75 (0.57–0.98) | 0.88 (0.62–1.24) | 0.60 (0.40–0.91) |
| > 300 | 0.72 (0.58–0.90) | 0.81 (0.61–1.08) | 0.70 (0.51–0.96) |
Note: CI = confidence interval, HR = hazard ratio, ref = reference group.
All models were stratified by age at start of renal replacement treatment. Stratification allowed different baseline hazards for each primary renal disease category and age category as appropriate.
Adjusted for all variables listed in the table.
Data for children from other ethnic minorities were not included because they did not meet the assumptions of proportional hazards.
Model was stratified by primary cause of renal disease.
After adjustment for confounders, children living more than 150 km from a pediatric renal care centre were less likely than those living closer to receive a transplant from any donor or from a deceased donor at any time after the start of renal replacement (Table 3). The influence of Aboriginal ethnicity on time to transplantation did not vary by distance to the nearest centre or by income quintile; all interaction terms between ethnicity and distance category, and between ethnicity and income quintile had p values greater than 0.05.
Graft survival
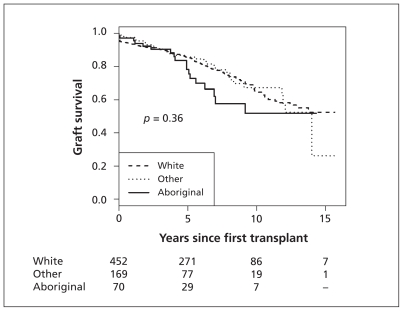
The median time to graft failure from first kidney transplant was 4.5 (IQR 1.9–5.5) years among Aboriginal children, 4.8 (IQR 0.9–7.7) years among white children and 3.8 (IQR 1.9–7.9) years among children of other ethnicity. The overall 5- and 10-year cumulative rates of graft survival by ethnic group are shown in Table 4. Figure 2 shows the Kaplan–Meier analysis of time to graft failure in the three groups.
Table 4:
Outcomes of patients, by ethnic group
| Outcome | Ethnic group | ||
|---|---|---|---|
| Aboriginal | White | Other* | |
| Graft† | n = 70 | n = 452 | n = 169 |
| Graft failure, no. (%) | 18 (25.7) | 113 (25.0) | 32 (18.9) |
| 5-year survival, % (95% CI) | 78.2 (67.2–91.0) | 85.8 (82.5–89.4) | 85.7 (79.7–92.2) |
| 10-year survival, % (95% CI) | 51.8 (36.0–74.4) | 65.3 (59.4–71.8) | 67.3 (56.7–79.9) |
| Patient† | n = 104 | n = 521 | n = 218 |
| Deaths, no. (%) | 15 (14.4) | 65 (12.5) | 27 (12.4) |
| 5-year survival, % (95% CI) | 91.3 (85.6–97.3) | 92.2 ( 89.8–94.7) | 90.8 (86.6–95.1) |
| 10-year survival, % (95% CI) | 84.8 (76.4–94.3) | 85.8 (82.2–89.6) | 86.8 (81.3–92.6) |
Note: CI = confidence interval.
Includes black, Asian, Indian subcontinent, Mid-east/Arabian, Pacific Islander, other or unknown.
Graft outcomes are for transplant recipients; patient data are for dialysis patients and transplant recipients.
Figure 2:
Kaplan–Meier analysis of graft survival after first kidney transplant, by ethnic group.
Patient survival
During the study period, 15 Aboriginal children (14.4%), 65 white children (12.5%) and 27 children of other ethnicity (12.4%) died (Table 4). The unadjusted mortality per 1000 patient-years was 21.9 (95% CI 10.8–33.0) among the Aboriginal children, 16.1 (95% CI 12.2–20.1) among the white children and 21.1 (95% CI 13.1–29.0) among the children of other ethnicity. Overall 5-and 10-year survival rates from the start of renal replacement treatment by ethnic group are shown in Table 4. There was no evidence of a difference in overall unadjusted survival between the three groups (log-rank p = 0.36). Adjustment for potential confounders in the association between survival and ethnicity was not performed because of the small number of deaths observed in the study groups.
Interpretation
We found that the time from start of renal replacement treatment to first kidney transplant was longer among Aboriginal children than among white children. After adjustment for confounders, Aboriginal children were 64% less likely to receive a transplant from a living donor and 38% less likely to receive a transplant from a deceased donor compared with white children who had the same time elapsed since start of dialysis. Aboriginal children had similar overall survival compared with the other ethnic groups.
The low rate of transplants from living donors among the Aboriginal children may explain in part the finding that Aboriginal children spent longer on dialysis than white children did. Rates of kidney donation from living donors have been reported to be lower among Aboriginal adults than among white adults.2,3 Various determinants of living donation, including awareness of organ donation in Aboriginal communities, medical suitability of relatives and culturally based perspectives influencing organ donation, need to be further addressed in the context of children.6,15–17
A slower process of transplant work-up or delay in initiation of transplant assessments may be key determinants of longer time to transplant observed among Aboriginal children.5,18 We observed that a higher proportion of Aboriginal children than of white children had glomerulonephritis or autoimmune disease as a cause of their end-stage renal disease, a finding that is consistent with a previous report from British Columbia.9 The time required to induce and maintain remission of primary disease before kidney transplant in patients with glomerulonephritis may have contributed to the delay in transplantation among Aboriginal children.
The prevalence of ABO blood groups among the Aboriginal and white children in our study were similar; therefore, we do not expect a systematic bias against organ allocation to Aboriginal children because of blood group incompatibility. Aboriginal children may be disadvantaged during organ allocation if there are differences between Aboriginal and non-Aboriginal children with respect to frequency of certain human leukocyte antigen (HLA) loci compared with the donor pool. Although pediatric patients are generally considered to have “high priority” in organ allocation algorithms in Canada and the level of priority based on HLA matching has diminished over time, HLA matching may still a play a role in some organ allocation algorithms.19 An evaluation of HLA “matchability” and its potential effect on time to transplant among Aboriginal children will be the subject of future study.
In a study of health outcomes of Aboriginal and white adults after the start of dialysis, Tonelli and colleagues found that the rate of kidney transplantation was lower among the Aboriginal adults and that this lower rate may have adversely affected their survival when receiving renal replacement treatment.4 Although we found lower rates of kidney transplantation among the Aboriginal children in our study, we did not find a difference in overall unadjusted patient survival between the three ethnic groups. We were unable to assess the impact of decreased likelihood of transplantation on survival using adjusted models because of the small number of deaths in the study groups.
Limitations
Our study has limitations. First, the Canadian Organ Replacement Register does not contain information on transplant wait list or the timing of transplant assessment. Therefore, we were unable to examine ineligibility for transplant, delays in referral for or completion of transplant assessment.
Second, ethnic background may have been misclassified for some patients. It is not possible to differentiate between status and non-status First Nations, Inuit and Métis, and there may be important ancestral or genetic differences among these population groups that influence risk and outcomes of renal disease. The “other” ethnic group is a heterogeneous mix of racial groups and thus, we are limited in our conclusions regarding outcomes in the other ethnic group.
Third, the Canadian Organ Replacement Register data are designed mainly for adults, and we did not have detailed comorbidity information relevant for pediatric cohorts. Usual adult co-morbidities are rare among pediatric patients with renal failure.
Fourth, our analysis did not account for regional variation in transplant wait times or changes in organ allocation policies for children over calendar time.
Conclusion
We found that the time from start of dialysis to first kidney transplant was longer among Aboriginal children than among white children. Further evaluation is necessary to examine individual and system barriers contributing to longer time to transplantation among Aboriginal children.
Acknowledgements
The authors thank Dr. John Gill and Yingbo Na of the Canadian Organ Replacement Register at the Canadian Institute for Health Information for their assistance in providing the dataset used in this study. This work was presented in abstract form at the American Society of Nephrology meeting, Nov. 16–21, 2010, in Denver, Colo. Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not those of the Canadian Institute for Health Information.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Contributors: All of the authors contributed to the study concept and design. Susan Samuel, Brenda Hemmelgarn and Marcello Tonelli obtained the dataset used in this study. Alberto Nettel-Aguirre and Andrea Soo directed and performed the data analysis. All of the authors contributed to the analysis and interpretation of the data. Susan Samuel drafted the manuscript and all of the coauthors reviewed the manuscript critically for important intellectual content. All of the authors approved the final version of the manuscript submitted for publication.
Funding: This study was funded by operating grants from the Alberta Children’s Hospital Foundation. Susan Samuel was supported by a Clinical Fellowship Award from the Alberta Heritage Foundation for Medical Research during the data-acquisition period of this study and recently received a Career Development Award from the Canadian Child Health Clinician Scientist Program. Bethany Foster holds a Chercheur-Boursier Clinicien award from the Fonds de la recherche en santé du Québec. Marcello Tonelli is supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease and by a Population Health Scholar Award from the Alberta Heritage Foundation for Medical Research. Todd Alexander is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research, a KRESCENT (Kidney Research Scientist Core Education and National Training Program) New Investigator Award and a Clinical Investigator Award from the Alberta Heritage Foundation for Medical Research. Brenda Hemmelgarn is supported by a New Investigator Award from the Canadian Institutes of Health Research and by a Population Health Investigator Award from Alberta Heritage Foundation for Medical Research.
The funding agencies did not participate in the analyses or influence the decision to submit the manuscript for publication.
References
- 1.Yeates KE. Aboriginal patients on the road to kidney transplantation: Is residence location a barrier? Kidney Int 2006;70:826–8 [DOI] [PubMed] [Google Scholar]
- 2.Yeates KE, Cass A, Sequist TD, et al. Indigenous people in Australia, Canada, New Zealand and the United States are less likely to receive renal transplantation. Kidney Int 2009;76:659–64 [DOI] [PubMed] [Google Scholar]
- 3.Yeates KE, Schaubel DE, Cass A, et al. Access to renal transplantation for minority patients with ESRD in Canada. Am J Kidney Dis 2004;44:1083–9 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Hemmelgarn B, Manns B, et al. Death and renal transplantation among Aboriginal people undergoing dialysis. CMAJ 2004;171:577–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonelli M, Chou S, Gourishankar S, et al. Wait-listing for kidney transplantation among Aboriginal hemodialysis patients. Am J Kidney Dis 2005;46:1117–23 [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Hemmelgarn B, Kim AK, et al. Association between residence location and likelihood of kidney transplantation in Aboriginal patients treated with dialysis in Canada. Kidney Int 2006;70:924–30 [DOI] [PubMed] [Google Scholar]
- 7.Weber CL, Rush DN, Jeffery JR, et al. Kidney transplantation outcomes in Canadian aboriginals. Am J Transplant 2006;6: 1875–81 [DOI] [PubMed] [Google Scholar]
- 8.Young TK, Kaufert JM, McKenzie JK, et al. Excessive burden of end-state renal disease among Canadian Indians: a national survey. Am J Public Health 1989;79:756–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda-Abedini M, Al-Alsheikh K, Hurley RM, et al. Outcome of kidney transplantation in Canadian Aboriginal children in the province of British Columbia. Pediatr Transplant 2009; 13:856–60 [DOI] [PubMed] [Google Scholar]
- 10.Samuel SM, Tonelli MA, Foster BJ, et al. Overview of the Canadian pediatric end-stage renal disease database. BMC Nephrol 2010;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Data quality study on the Canadian Organ Replacement Register. Ottawa (ON): Canadian Institute for Health Information; 2009 [Google Scholar]
- 12.Moist LM, Richards HA, Miskulin D, et al. A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol 2011;6:813–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26 [Google Scholar]
- 14.van Buuren S, Oudshoorn CGM. Multivariate Imputation by Chained Equations: MICE v1.0 user’s manual. Leiden (Netherlands): Netherlands Organziation for Applied Scientific Research; 2000. Available: http://web.inter.nl.net/users/S.van.Buuren/mi/docs/Manual.pdf (accessed 2011 May 9). [Google Scholar]
- 15.Cass A, Cunningham J, Anderson K, et al. Decision-making about suitability for kidney transplantation: results of a national survey of Australian nephrologists. Nephrology (Carlton) 2007;12:299–304 [DOI] [PubMed] [Google Scholar]
- 16.Cass A, Cunningham J, Snelling P, et al. Renal transplantation for Indigenous Australians: identifying the barriers to equitable access. Ethn Health 2003;8:111–9 [DOI] [PubMed] [Google Scholar]
- 17.McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton) 2003;8:42–8 [DOI] [PubMed] [Google Scholar]
- 18.Anderson K, Cunningham K, Devitt J, et al. They really want to go back home, they hate it here: the importance of place in Canadian health professionals’ views on the barriers facing Aboriginal patients accessing kidney transplants. Health Place 2009;15:390–3 [DOI] [PubMed] [Google Scholar]
- 19.Kidney allocation in Canada: a Canadian forum. October 25–27, 2006; Toronto, Ontario: report and recommendations. Edmonton (AB): Canadian Council for Donation and Transplantation; 2007. Available: www.ccdt.ca/english/publications/final-pdfs/Kidney_Allocation_FINAL.pdf (accessed 2011 May 6). [Google Scholar]