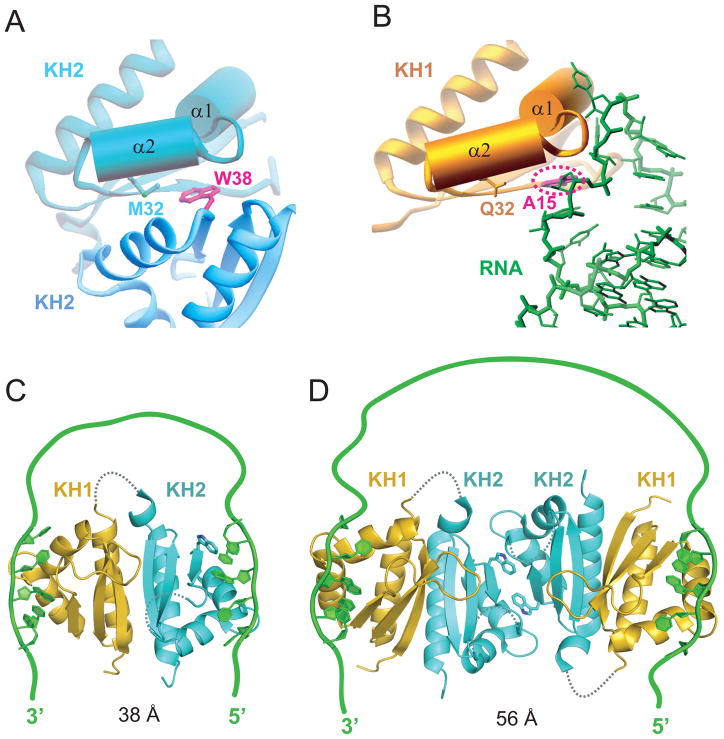
Figure 9. Models for Interaction of Nova KH1/2 Domains with Wild-type RNA Targets through a RNA Looping Mechanism.
(A) The KH2-KH2 interaction between symmetry-related molecules in the crystal of the KH1/2-RNA hairpin complex. Note the juxtaposition of Met32 and Trp38 within van der Waals contact across the interface.
(B) The KH1 (gold) - RNA (green) interface in the KH1/2-RNA hairpin complex. Note the juxtaposition of Gln32 on KH1 and A15 on the RNA within van der Waals contact across the intermolecular interface.
(C) Model where Nova-1 KH1/2 monomer (based on ultracentrifugation data in solution) is targeted by a RNA containing a pair of sequence elements capable of targeting KH1 (with high affinity) and KH2 (most likely with lower affinity) and separated by a linker segment of sufficient length. The stoichiometry of this complex is 1:1 Nova-1 KH1/2:RNA.
(D) Model of Nova-1 KH1/2 based on the x-ray structure of the complex (Figure 2A), where the RNA-binding surfaces of the KH2 domains pack against each other in the crystal lattice. The target RNA contains a pair of sequence elements capable of targeting KH1 with high affinity and separated by a longer linker segment of sufficient length. The stoichiometry of this complex is 2:1 Nova-1 KH1/2:RNA.