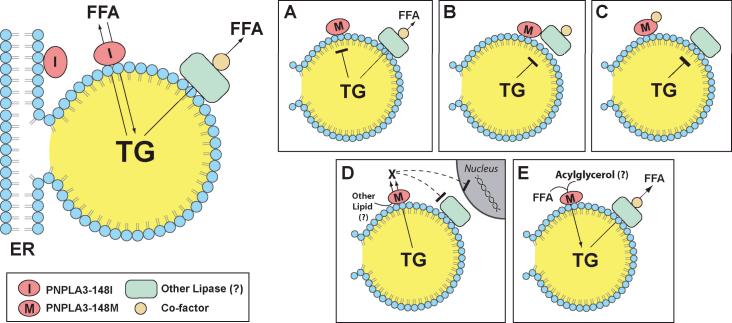
Figure 1.
Potential mechanistic links between PNPLA3-I148M and NAFLD. PNPLA3 is associated with both the endoplasmic reticulum (ER) and lipid droplets in hepatocytes and exhibits triglyceride hydrolase and transacylation activity in vitro (top left) (13, (16). In the fed state, the protein may serve to liberate free fatty acids (FFA) from triglyceride (TG) contained within lipid droplets. Alternatively, PNPLA3 could be involved in the conversion of acylglycerols to triglycerides, thereby promoting TG deposition. Substitution of methionine for isoleucine at residue 148 (PNPLA3-I148M) abolishes TG hydrolase activity (15), suggesting that PNPLA3-I148M is a loss-of-function mutation (A). However, overexpression of PNPLA3-I148M in the liver of mice results in steatosis (15). These findings are more consistent with the variant having a gain-of-function. The mutant protein may interfere with the action of another lipase (B), or sequester a necessary co-factor required for maintenance of triglyceride homeostasis in liver (C). Alternatively, expression of the mutant enzyme may generate a new signaling molecule that inhibits lipolysis (D) or promotes triglyceride formation (E).