Table 1.
Initial screenings of Au(I)/Au(III)-catalyzed Sonogashira couplinga.
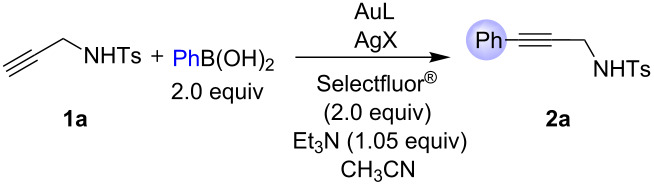 | ||||
| Entry | AuL (5 mol %) | AgX (mol %) | Time (h) | Yield (%)b |
| 1 | Ph3PAuCl | — | 18 | trace |
| 2 | Ph3PAuCl | AgOTf (5) | 22 | 56 |
| 3 | — | AgOTf (5) | 24 | 0 |
| 4 | AuCl | AgOTf (5) | 24 | 21 |
| 5 | AuCl3 | AgOTf (15) | 24 | 37c |
| 6d | Ph3PAuCl | AgOTf (5) | 22 | 0 |
| 7e | Ph3PAuCl | AgOTf (5) | 22 | 41 |
| 8 | Ph3PAuCl | AgBF4 (5) | 10 | 65 |
| 9 | Ph3PAuCl | AgSbF4(5) | 10 | 62 |
| 10 | (XPhos)AuCl | AgOTf (5) | 24 | 0 |
| 11f | dppm(AuCl)2 | AgOTf (5) | 16 | 83c |
| 12f | dppm(AuBr)2 | — | 16 | trace |
| 13f,g | Ph3PAuCl | AgOTf (5) | 12 | 72 (73c) |
| 14f | Ph3PAuCl | AgBF4 (5) | 12 | 75 (80c) |
aReaction conditions: The reaction was carried out by using 1a (0.4 mmol) and phenylboronic acid (0.8 mmol, 2.0 equiv), and 1.05 equiv of Et3N in 2 mL of CH3CN stirred at room temperature. bIsolated yields. cYield determined by 1H NMR with dibromomethane as an internal standard. dSelectfluor® (0 equiv). ePhB(OH)2 (1.5 equiv). fUnder an atmosphere of nitrogen (N2). gThe reaction temperature was 50 °C.
