Abstract
Context:
Anorexia nervosa is complicated by severe bone loss and clinical fractures. Mechanisms underlying bone loss in adults with anorexia nervosa include increased bone resorption and decreased formation. Estrogen administration has not been shown to prevent bone loss in this population, and to date, there are no approved, effective therapies for this comorbidity.
Objective:
To determine whether antiresorptive therapy with a bisphosphonate alone or in combination with low-dose transdermal testosterone replacement would increase bone mineral density (BMD) in women with anorexia nervosa.
Design and Setting:
We conducted a12-month, randomized, placebo-controlled study at a clinical research center.
Study Participants:
Participants included 77 ambulatory women with anorexia nervosa.
Intervention:
Subjects were randomized to risedronate 35 mg weekly, low-dose transdermal testosterone replacement therapy, combination therapy or double placebo.
Main Outcome Measures:
BMD at the spine (primary endpoint), hip, and radius and body composition were measured by dual-energy x-ray absorptiometry.
Results:
Risedronate increased posteroanterior spine BMD 3%, lateral spine BMD 4%, and hip BMD 2% in women with anorexia nervosa compared with placebo in a 12-month clinical trial. Testosterone administration did not improve BMD but increased lean body mass. There were few side effects associated with either therapy.
Conclusions:
Risedronate administration for 1 yr increased spinal BMD, the primary site of bone loss in women with anorexia nervosa. Low-dose testosterone did not change BMD but increased lean body mass.
Anorexia nervosa is a primary psychiatric disease resulting in chronic starvation and serious medical complications, including severe bone loss in nearly 50% of affected adult women (1, 2). Bone loss affects the spine preferentially and is characterized by both increased bone resorption and decreased formation (1–3). There are few effective, and no U.S. Food and Drug Administration-approved, therapies for low bone mass for this population. Estrogen/progestin therapy is ineffective in preventing or reversing bone loss in adults with anorexia nervosa (4, 5), despite its established effectiveness in preventing bone loss in postmenopausal women. In one report, recombinant IGF-I therapy increased bone formation and bone mineral density (BMD) when administered for 9 months in combination with oral contraceptives but did not increase BMD to normal (4).
Oral bisphosphonates have been shown in numerous large studies to be a highly effective antiresorptive therapy, resulting in significant increases in BMD and reductions in fracture risk in postmenopausal women (6–10). Bisphosphonates are also an effective treatment for osteoporosis in pre- and postmenopausal women receiving glucocorticoids (11). It is unknown whether antiresorptive therapy would be effective in the setting of profound malnutrition. There are few data examining the effectiveness of such therapy in anorexia nervosa, and no placebo-controlled studies of adults with this disease have been published. In a small open-label study of risedronate administration to 10 adults with anorexia nervosa, we demonstrated a mean 4.1% increase in spine BMD at 6 months and 4.9% at 9 months compared with baseline and 5.6% increase at 6 months and 5.9% increase at 9 months compared with historical controls (12). In a 12-month, randomized, placebo-controlled study of alendronate 10 mg daily in 32 adolescents with anorexia nervosa, Golden et al. (13) demonstrated mean increases in femoral neck and lumbar spine BMD of 4.4 and 3.5%, respectively, compared with baseline. However, there was no significant increase compared with placebo group. It is not clear whether the conflicting results reflect differential age-related effects, particularly given differences in normal bone physiology in children vs. adults, the high rate of weight gain in the adolescent subjects receiving placebo in the Golden et al. (13) study, or other factors. Therefore, a randomized, placebo-controlled study of bisphosphonate therapy is needed to establish whether bisphosphonate therapy is effective in adult women with anorexia nervosa.
Androgen receptors are found on osteoblasts (14), and androgen administration is anabolic to bone in hypogonadal men (15, 16). Small randomized, controlled studies in postmenopausal and hypopituitary women have demonstrated that low doses of androgens, in a range that is physiological for women, increase BMD (17–19). We have previously demonstrated relative testosterone deficiency in women with anorexia nervosa (20). In addition, we have shown in a 3-wk, randomized, placebo-controlled study that low-dose transdermal testosterone replacement stimulates bone formation in such women (21).
Because bone loss in anorexia nervosa is characterized by both increased resorption and decreased formation (3), we hypothesized that maximal gains in bone mass would be accomplished by coadministration of a bisphosphonate and testosterone replacement. In addition, our previous study of recombinant human IGF-I and estrogen coadministration provided proof of principle of the potential efficacy of combined therapy with an antiresorptive and anabolic agent in young women with anorexia nervosa (4). Therefore, in 77 women with anorexia nervosa, we determined whether risedronate, testosterone, or combination therapy increases BMD in this population in a 12-month placebo-controlled study.
Subjects and Methods
Study participants
The study was approved by the Partners Healthcare, Inc., Review Board, and written informed consent was obtained from all subjects. Potential participants were recruited from referring healthcare providers and through advertisements. All study participants were ambulatory and met all psychiatric and weight-related DSM IV criteria for the diagnosis of anorexia nervosa and had BMD Z-scores below 1.0 in at least one skeletal site. Exclusion criteria included serum alanine aminotransferase (ALT) or creatinine more than three times the upper limit of normal, conditions or use of medications (other than oral contraceptives) known to affect bone metabolism, potassium below 3.0 meq/liter, active substance abuse, history of esophageal ulcers or difficulty swallowing, pregnancy, or lactation.
Protocol
The study was a 12-month, double-blind, randomized, placebo-controlled protocol performed on the Massachusetts General Hospital Clinical Research Center. At baseline (before initiation of therapy), a history and physical were performed. Research bionutritionists measured each participant's weight in a gown, calculated body mass index (BMI), measured frame size, calculated percentage of ideal body weight, and assessed activity level using the Modifiable Activity Questionnaire, which assesses the number of hours per week spent in leisure and occupational activity over the previous year (22). Blood was drawn for testosterone, free testosterone, ALT, aspartate aminotransferase (AST), N-terminal propeptide of type 1 procollagen (P1NP), and type I collagen C-telopeptide (CTX), dual energy x-ray absorptiometry (DXA) was performed at the spine, hip, and radius to assess baseline BMD and of the whole body to assess body composition, and bioelectric impedance analysis was performed to assess total body water. Facial and body hair were assessed with the Lorenzo hirsutism scoring system, a validated rating system that characterizes the density and distribution of pigmented hair on the upper lip, chin, chest, thighs, abdomen, and forearms (23). Hair growth at each location is scored from 0–4, for a total score of 0–24. Acne was assessed by physical exam and by interview of each study subject at each study visit.
After baseline evaluation, subjects were randomized to receive 1) risedronate 35 mg (Procter and Gamble Pharmaceuticals, Cincinnati, OH) weekly plus a placebo patch, 2) testosterone 150 μg daily patch (Procter and Gamble Pharmaceuticals) plus a weekly placebo pill, 3) risedronate 35 mg weekly plus testosterone 150 μg daily, or 4) double placebo for 12 months. Randomization was performed by the Massachusetts General Hospital Research Pharmacy and was stratified for current oral contraceptive use.
Follow-up visits were performed at 1, 3, 6, 9, and 12 months after the baseline evaluation, and urine pregnancy tests were required monthly. BMD and body composition were assessed at 6, 9, and 12 months after randomization. Markers of bone metabolism, total testosterone, free testosterone, and ALT were measured, and hirsutism (by the Lorenzo Hirsutism Scoring System) and acne were assessed 1, 3, 6, 9, and 12 months after randomization. Activity level was assessed by the Modifiable Activity Questionnaire 12 months after the baseline evaluation. Subjects continued to receive ongoing care from their own individualized treatment teams throughout the study.
The initial testosterone dose was 150 μg daily (one 150-μg patch and one placebo patch), which was increased to 300 μg daily (two 150-μg patches) in subjects whose free testosterone levels remained below the median on the initial dose (n = 25). The dose was decreased to 150 μg daily in subjects receiving 300 μg daily if one free testosterone level was more than two times the upper limit of normal or two free testosterone levels were more than 1.3 times the upper limit of normal. Testosterone was discontinued in subjects receiving 150 μg daily based on the same predetermined parameters. Dose titration was performed by an unblinded healthcare provider who determined whether testosterone doses should be increased or decreased, and sham dose-adjusted study subjects who were randomized to placebo to maintain double blinding. All subjects were provided with a standard multivitamin containing 400 IU vitamin D daily. A bionutritionist assessed subjects' dietary calcium intakes, and subjects were given calcium supplements in an amount necessary to provide a calcium intake of 1200 mg daily.
Eighteen of the 77 study participants did not complete the study; 16 dropped out due to personal and/or scheduling issues, and two were discontinued from the study by study investigators because of psychiatric or medical issues, e.g. extreme decreases in weight. Data from one outlier, a subject who experienced a 24-kg weight gain between the baseline and 6-month visit, were excluded from analysis.
Body composition evaluation
Fat mass and fat-free mass were measured by DXA (Hologic 4500; Hologic Inc., Waltham, MA), with a percent accuracy error for BMD below 1% (24), for body fat mass of 1.4%, and for fat-free mass of 1.5% (25). Hologic normative data were used to determine Z-scores at all skeletal sites, except the hip, for which National Health and Nutrition Examination Survey data (26) were used. Total body water was measured using bioimpedance analysis (Quantum II, Bioelectric Impedance Analysis Instrument with Cyprus version 2.7 software; RJL Systems, Clinton Township, MI).
Biochemical analyses
Serum and plasma samples were collected and stored at −80 C. Testosterone was measured by liquid chromatography mass spectroscopy (Esoterix Inc., Calabassas Hills, CA) with a normal female range of 10–55 ng/dl. Percent free testosterone was measured by equilibrium dialysis (Esoterix), with a minimum reportable free fraction of 0.1%. Free testosterone was calculated as the product of total testosterone and percent free testosterone. The normal female range for free testosterone is 1.1–6.3 pg/ml. ALT, AST, and creatinine were measured using standard automated methods in the Massachusetts General Hospital clinical chemistry laboratories in the Department of Pathology.
Statistical analysis
The study was powered to detect an increase in posteroanterior (PA) spine BMD with risedronate (12) and testosterone (17) of 0.02 gm/cm2·yr with an n of 80. Data were naturally log-transformed before statistical analysis was performed. Baseline mean clinical characteristics were compared by ANOVA, and data are presented as means ± sd. For the longitudinal analyses, factorial analyses were performed to determine the effects of risedronate and testosterone on bone and body composition endpoints using repeated-measures analysis of covariance using the log-baseline measure as a covariate. The differences in log-transformed values are reported as percent change, which they closely approximate, and 95% confidence intervals (CI) are presented. In addition, multivariate regression models were constructed to determine whether the following baseline (i.e. pretreatment) variables predicted response to therapy: 1) anorexia nervosa subtype (restrictive vs. bulimic), 2) presence or absence of menses, 3) use of oral contraceptives, 4) BMD, 5) weight, 6) CTX, 7) P1NP, and 8) estrogen status. Univariate regression models were constructed to investigate whether changes in androgen levels predicted changes in BMD, body composition, and bone metabolism parameters, and Spearman coefficients are reported. Fisher's exact testing was used to compare rates of side effects between groups. Statistical significance was defined as a two-tailed P value ≤0.05.
Results
Baseline clinical characteristics
Baseline and 12-month clinical characteristics are presented in Table 1 and were all comparable between the groups. Seventy-seven subjects were randomized to four treatment groups: risedronate 35 mg weekly, transdermal testosterone 150 μg daily, combination therapy, or double placebo. At baseline, the four groups were of comparable mean age and with comparable mean BMD and BMD Z-scores at all sites and of similar body composition.
Table 1.
Baseline clinical characteristics
| Double placebo (n = 18) | Risedronate/testosterone (n = 20) | Risedronate (n = 20) | Testosterone (n = 19) | |
|---|---|---|---|---|
| Age (yr) | 26.9 ± 7.2 | 25.2 ± 6.2 | 25.3 ± 6.3 | 27.1 ± 7.3 |
| Duration of anorexia nervosa (yr) | 5.2 ± 4.3 | 6.3 ± 6.8 | 5.1 ± 5.8 | 6.6 ± 5.5 |
| Oral contraceptive use (%) | 28 | 25 | 25 | 26 |
| Endogenous menses (%) | 13 | 26 | 29 | 21 |
| Bulimic subtype (%) | 39 | 40 | 50 | 26 |
| Activity (MET-h/wk) | 19 ± 12 | 15 ± 11 | 22 ± 16 | 19 ± 12 |
| Weight (kg) | 47.6 ± 5.2 | 47.9 ± 5.4 | 47.2 ± 5.6 | 47.8 ± 5.5 |
| % IBW | 78.9 ± 5.7 | 78.7 ± 7.1 | 78.8 ± 5.7 | 78.7 ± 7.5 |
| BMI (kg/m2) | 17.9 ± 1.2 | 17.8 ± 1.4 | 17.6 ± 1.2 | 17.5 ± 1.8 |
| Lean body mass (kg) | 37.0 ± 4.0 | 37.4 ± 4.3 | 36.7 ± 4.7 | 37.7 ± 4.3 |
| % Body fat | 19.2 ± 4.7 | 18.9 ± 4.1 | 19.2 ± 4.8 | 17.9 ± 5.1 |
| PA spine Z-score | −1.7 ± 0.8 | −1.5 ± 0.9 | −1.6 ± 0.8 | −1.5 ± 1.2 |
| Lateral spine Z-score | −1.7 ± 1.0 | −1.7 ± 1.1 | −1.4 ± 0.6 | −1.2 ± 0.9 |
| Hip Z-score | −1.4 ± 0.6 | −1.4 ± 0.7 | −1.2 ± 1.1 | −1.1 ± 1.0 |
| Radius Z-score | −0.6 ± 1.1 | −0.4 ± 0.8 | −0.6 ± 1.0 | −0.5 ± 0.9 |
| CTX (ng/ml) | 0.61 ± 0.37 | 0.66 ± 0.36 | 0.63 ± 0.40 | 0.53 ± 0.33 |
| P1NP (ng/ml) | 46.8 ± 21.8 | 53.1 ± 23.9 | 57.9 ± 42 | 39.9 ± 17.8 |
IBW, Ideal body weight; MET, metabolic equivalent.
Hormone levels
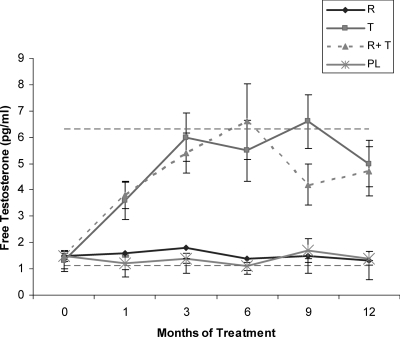
Free testosterone levels increased in women randomized to receive testosterone compared with those who were randomized to receive placebo patches (Fig. 1).
Fig. 1.
Serum free testosterone increased in women randomized to receive testosterone compared with those who were randomized to receive placebo patches. Horizontal dashed lines delineate the normal female range. R, Risedronate; T, testosterone; PL, double placebo. *, P < 0.05.
Bone mineral density
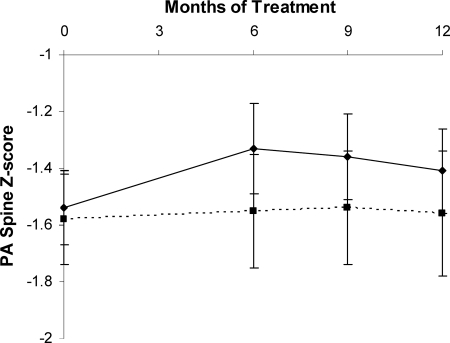
Risedronate increased PA spine BMD 3.2% (95% CI = 1.8–4.6%) compared with placebo (P < 0.0001) (Z-scores shown, Fig. 2), lateral spine BMD 3.8% (95% CI = 1.9–5.6%) compared with placebo (P = 0.0002), and hip BMD 1.9% (95% CI = 0.4–3.4%) compared with placebo (P = 0.013) over the 12-month treatment period. Risedronate had no significant effect on radial BMD. Testosterone administration did not have a significant effect on BMD at any site. There was no significant interaction between testosterone and risedronate on the primary efficacy measures; i.e. there was no significant difference in the response of BMD to risedronate between the patients receiving testosterone coadministration and those receiving placebo patches.
Fig. 2.
PA spine BMD increased in women receiving risedronate (solid line) over a 12-month period compared with those receiving placebo (dotted line) (P < 0.0001). Z-scores are shown.
Markers of bone metabolism
Risedronate decreased CTX 41% (95% CI = 17–65%) (P = 0.002) and P1NP 33% (95% CI = 19–46%) (P < 0.001) compared with placebo over the 12-month treatment period. Testosterone had no effect on CTX or P1NP over the 12-month period.
Weight and body composition
There was no significant change in weight in any group or difference between the groups in weight change over the 12-month period (P = 0.50). Testosterone increased lean body mass 1.9% (95% CI = 0.2–3.6%) compared with placebo over the 12-month period (P = 0.037) but had no significant effect on total body water or fat mass.
Predictors of response
Subjects with a bulimic subtype of anorexia nervosa did not experience as robust a suppression of CTX to risedronate as did those with a restrictive subtype (P = 0.039); however, there was no statistically significant effect of anorexia nervosa subtype on response of BMD to risedronate. Oral contraceptive use resulted in a less robust response to risedronate of BMD at the lateral spine (P = 0.030). Neither anorexia nervosa subtype, oral contraceptive use, nor activity level affected response of any other variable to therapy. Neither baseline BMD, baseline weight, nor presence vs. absence of menses was a significant predictor of response of BMD to therapy at any skeletal site. Pretreatment estrogen status (amenorrheic and not receiving oral contraceptive pills vs. those with menses or receiving oral contraceptive pills) did not predict body composition, bone turnover marker, or BMD response.
A higher pretreatment state of bone resorption, as measured by CTX, predicted a greater response to risedronate over the 12-month period at the PA spine BMD (P = 0.014), lateral spine BMD (P = 0.001), and total hip BMD (P = 0.047). Pretreatment P1NP level did not predict response to therapy. Change in CTX over the 12-month period was inversely associated with change in PA spine BMD within the risedronate alone group (r = −0.58; P = 0.037).
Correlates of change in androgen levels
In the testosterone and double-placebo groups combined, increases in total testosterone (r = 0.61; P = 0.0007) and free testosterone (r = 0.48; P = 0.012) were associated with increases in lean body mass. Increases in free testosterone were associated with an increase in P1NP levels at 1 month (r = 0.42; P = 0.021) and 3 months (r = 0.41; P = 0.027) but not at 6, 9, or 12 months. The increase in total testosterone at 3 months was associated with an increase in P1NP (r = 0.42; P = 0.020). There was no association between change in testosterone or free testosterone and CTX at any time point. Changes in total testosterone (r = 0.45; P = 0.017) and free testosterone (r = 0.49; P = 0.009) over the 12-month period were associated with increases in hip bone area. There was no association between change in free testosterone levels and bone area at other skeletal sites or BMD at any site.
Side effects
There was no change in mean Lorenzo hirsutism scores over the 12-month study period in testosterone users compared with non-testosterone users. One study participant in the placebo group, and none in the testosterone group, discontinued patch use due to perceived increased hirsutism and mood changes. There was no effect of either risedronate or testosterone on ALT or asparate aminotransferase levels. Comparable numbers of women receiving testosterone and placebo patches reported mild irritation at the patch site (testosterone, n = 20; placebo, n = 15), mild increase in oily skin or acne (testosterone, n = 21; placebo, n = 25), or a mild increase in body hair (testosterone, n = 13; placebo, n = 6). A comparable number of women in the risedronate and placebo tablet groups reported myalgias (risedronate, n = 4; placebo, n = 3). More women in placebo than risedronate reported reflux symptoms (risedronate, n = 4; placebo, n = 11; P = 0.02). None of these side effects was severe enough to prompt discontinuation from the study on the part of any study subject.
Discussion
In this 12-month, randomized, placebo-controlled study, we demonstrate that risderonate increases spine and hip BMD in women with anorexia nervosa. This is an important finding given the lack of effectiveness of other agents, including estrogen, to prevent or reverse bone loss in adults with anorexia nervosa, who are at increased risk for fractures, despite young age (2, 27, 28). Whether bisphosphonates should be routinely prescribed to women with anorexia nervosa to prevent or reverse bone loss is unclear, and it should be noted that they are not approved by the U.S. Food and Drug Administration for this indication at this time. In addition, although the few published reports on the use of bisphosphonates during pregnancy are reassuring (29, 30), there are few published data addressing this issue. Therefore, bisphosphonates should be prescribed with caution in a young woman and only under the supervision of a skeletal health specialist.
The magnitude of the residronate effect compares favorably with other therapies for BMD tested and found not to be effective in adults with anorexia nervosa, such as oral estrogen (5). We demonstrate an increase of 3% in a 12-month clinical trial at the lumbar spine in women with anorexia nervosa. Studies largely in postmenopausal women have shown a 4.5% (95% CI = 4.1–5.0) increase over 1.5–3 yr (31). The resistance of the chronic undernutrition state, in which therapies that are effective in other populations, e.g. estrogen, have been shown to be ineffective in women with anorexia nervosa is mostly unexplained, and predictors of response to therapy in the undernourished state have not been identified. Our analyses suggest that the degree of response to risedronate is largely unaffected by baseline weight, degree of bone loss, anorexia nervosa subtype (restrictive or bulimic), presence or absence of menses, or use of oral contraceptives. The implications of the two exceptions are unclear. We found that bulimic subtype predicted a less robust antiresorptive response to risedronate therapy than restrictive subtype, but our analyses did not reveal an effect of subtype on BMD at any site. Oral contraceptive use predicted less of an increase in lateral spine BMD but not at other skeletal sites. Whether in a larger study we would have observed that anorexia nervosa subtype or oral contraceptive use had more consistent effects on the outcome is unknown.
Low-dose testosterone administration increased lean body mass over the 12-month period, and acutely stimulated bone formation but did not increase BMD in women with anorexia nervosa. Our findings of a positive association between the increase in androgen levels and the increase in a marker of bone formation are consistent with our previous report demonstrating that a 3-wk course of low-dose testosterone increased bone formation in women with anorexia nervosa (21). However, we demonstrate in this longer study that the acute increase is not sustained. Increases in BMD were not achieved with low-dose testosterone therapy, although increases in androgen levels were associated with increases in hip bone area.
Whether a longer duration of therapy might have been more effective is unknown. The increase in hip bone area suggests that testosterone may stimulate the formation of new bone, which before being mineralized may not increase BMD as measured by DXA, as has been shown with other anabolic therapies. For example, early studies of GH therapy of less than 18 months duration showed no increase in BMD, and some demonstrated a slight decrease (32). This is in contrast to studies of 18 months duration or more, which afforded sufficient time for mineralization of newly formed bone and consistently demonstrated increases in BMD (33–35). The fact that an increase in bone area in response to testosterone administration was observed preferentially at the hip is consistent with our previous data, which suggest preferential effects of androgens at the hip (18, 36). An increase in hip bone area may be evidence of an anabolic effect on cortical bone, which may be beneficial, particularly for women with anorexia nervosa who are hyperexercisers. Additional studies investigating the differential effects of testosterone on cortical vs. trabecular microarchitecture and bone strength would be of interest.
The effects of testosterone to increase lean body mass may be important both as a strategy to preserve muscle mass and because of its potential to improve bone mass, although the latter is a theoretical benefit, because we did not detect an effect of testosterone on BMD in our study. Caloric restriction in women with anorexia nervosa is known to be associated with loss of muscle in addition to fat mass. In addition, lean body mass is a known important determinant of BMD, more important than BMI or fat mass in anorexia nervosa (37, 38). Of note, increases in lean body mass were achieved with free testosterone levels within the normal female range and with few side effects.
Our study results suggest positive effects of risedronate on bone in females with anorexia nervosa, with few side effects. We have reported impaired trabecular microarchitecture in women with anorexia nervosa using computed tomography (39, 40), and additional studies are warranted to determine the effects of therapeutic interventions on bone microarchitecture in this population. Another important advance that occurred since the initiation of this study was the demonstration that sequential anabolic therapy followed by consolidation antiresorptive therapy effectively increases BMD more than monotherapy in the specific case of PTH followed by a bisphosphonate (41). In contrast, concomitant administration of these two therapies was no more effective than PTH alone (42). This raises the question of whether sequential therapy with testosterone or another anabolic therapy followed by risedronate might have been more effective than the combination strategy we pursued. Another limitation of our study was that our dropout rate was higher than in our previous studies in this population, but it should be noted that it is substantially lower than in many studies in this patient population, which is known to be extremely difficult to recruit and maintain in studies, due to a variety of psychiatric issues (43).
In conclusion, our data demonstrated that a 1-yr course of the bisphosphonate risedronate was effective at increasing BMD in women with anorexia nervosa. Low-dose physiological testosterone administration increased lean body mass and acutely stimulated bone formation but did not increase BMD during the 1-yr course of the study. Whether a longer duration or a higher starting dose would have resulted in an increase in BMD is unknown. Additional studies are needed to determine strategies to optimize therapy for the severe bone loss observed in this young population at a significantly increased risk for fractures.
Acknowledgments
We thank the nurses and bionutritionists of the Massachusetts General Hospital Clinical Research Center and the patients who participated in the study.
This work was supported in part by National Institutes of Health Grants R01 DK052625, MO1 RR01066, and ULI RR0257801. Study drugs were provided for the study at no cost by Procter and Gamble Pharmaceuticals.
Disclosure Summary: The authors have no relevant conflicts to declare.
Footnotes
- ALT
- Alanine aminotransferase
- AST
- aspartate aminotransferase
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- CTX
- type I collagen C-telopeptide
- DXA
- dual-energy x-ray absorptiometry
- PA
- posteroanterior
- P1NP
- N-terminal propeptide of type 1 procollagen.
References
- 1. Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. 2000. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 133:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. 2005. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 165:561–566 [DOI] [PubMed] [Google Scholar]
- 3. Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. 1996. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab 81:3864–3870 [DOI] [PubMed] [Google Scholar]
- 4. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. 2002. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 87:2883–2891 [DOI] [PubMed] [Google Scholar]
- 5. Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. 1995. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80:898–904 [DOI] [PubMed] [Google Scholar]
- 6. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY. 2001. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340 [DOI] [PubMed] [Google Scholar]
- 7. Schott AM, Dargent-Molina P, Meunier PJ. 2001. The effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:1721. [PubMed] [Google Scholar]
- 8. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. 1996. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541 [DOI] [PubMed] [Google Scholar]
- 9. Ensrud KE, Black DM, Palermo L, Bauer DC, Barrett-Connor E, Quandt SA, Thompson DE, Karpf DB. 1997. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med 157:2617–2624 [PubMed] [Google Scholar]
- 10. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. 1998. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082 [DOI] [PubMed] [Google Scholar]
- 11. de Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, Buskens E, de Laet CE, Oostveen AC, Geusens PP, Bruyn GA, Dijkmans BA, Bijlsma JW. 2006. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355:675–684 [DOI] [PubMed] [Google Scholar]
- 12. Miller KK, Grieco KA, Mulder J, Grinspoon S, Mickley D, Yehezkel R, Herzog DB, Klibanski A. 2004. Effects of risedronate on bone density in anorexia nervosa. J Clin Endocrinol Metab 89:3903–3906 [DOI] [PubMed] [Google Scholar]
- 13. Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, Hertz S, Shenker IR. 2005. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 90:3179–3185 [DOI] [PubMed] [Google Scholar]
- 14. Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. 1997. The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82:3493–3497 [DOI] [PubMed] [Google Scholar]
- 15. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG, Jr, Strom BL. 1999. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 84:1966–1972 [DOI] [PubMed] [Google Scholar]
- 16. Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. 1996. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81:4358–4365 [DOI] [PubMed] [Google Scholar]
- 17. Davis SR, McCloud P, Strauss BJ, Burger H. 1995. Testosterone enhances estradiol's effects on postmenopausal bone density and sexuality. Maturitas 21:227–236 [DOI] [PubMed] [Google Scholar]
- 18. Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A. 2006. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 91:1683–1690 [DOI] [PubMed] [Google Scholar]
- 19. Barrett-Connor E, Young R, Notelovitz M, Sullivan J, Wiita B, Yang HM, Nolan J. 1999. A two-year, double-blind comparison of estrogen-androgen and conjugated estrogens in surgically menopausal women. Effects on bone mineral density, symptoms and lipid profiles. J Reprod Med 44:1012–1020 [PubMed] [Google Scholar]
- 20. Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A. 2007. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 92:1334–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller KK, Grieco KA, Klibanski A. 2005. Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab 90:1428–1433 [DOI] [PubMed] [Google Scholar]
- 22. Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. 1995. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol 142:191–201 [DOI] [PubMed] [Google Scholar]
- 23. Moncada E. 1970. Familial study of hirsutism. J Clin Endocrinol Metab 31:556–564 [DOI] [PubMed] [Google Scholar]
- 24. Barthe N, Braillon P, Ducassou D, Basse-Cathalinat B. 1997. Comparison of two Hologic DXA systems (QDR 1000 and QDR 4500/A). Br J Radiol 70:728–739 [DOI] [PubMed] [Google Scholar]
- 25. Mazess RB, Barden HS, Bisek JP, Hanson J. 1990. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51:1106–1112 [DOI] [PubMed] [Google Scholar]
- 26. National Center for Health Statistics, Center for Disease Control and Prevention National Health and Nutrition Examination Survey III. cdc.gov/nchs/nhanes.htm
- 27. Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. 1991. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 265:1133–1138 [PubMed] [Google Scholar]
- 28. Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. 1999. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 74:972–977 [DOI] [PubMed] [Google Scholar]
- 29. Djokanovic N, Klieger-Grossmann C, Koren G. 2008. Does treatment with bisphosphonates endanger the human pregnancy? J Obstet Gynaecol Can 30:1146–1148 [DOI] [PubMed] [Google Scholar]
- 30. Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, Koren G, Ito S. 2009. Pregnancy outcome following in utero exposure to bisphosphonates. Bone 44:428–430 [DOI] [PubMed] [Google Scholar]
- 31. Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, Robinson V, Shea B, Wells G, Guyatt G. 2002. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 23:517–523 [DOI] [PubMed] [Google Scholar]
- 32. Hansen TB, Brixen K, Vahl N, Jørgensen JO, Christiansen JS, Mosekilde L, Hagen C. 1996. Effects of 12 months of growth hormone (GH) treatment on calciotropic hormones, calcium homeostasis, and bone metabolism in adults with acquired GH deficiency: a double blind, randomized, placebo-controlled study. J Clin Endocrinol Metab 81:3352–3359 [DOI] [PubMed] [Google Scholar]
- 33. Baum HB, Biller BM, Finkelstein JS, Cannistraro KB, Oppenhein DS, Schoenfeld DA, Michel TH, Wittink H, Klibanski A. 1996. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med 125:883–890 [DOI] [PubMed] [Google Scholar]
- 34. Snyder PJ, Biller BM, Zagar A, Jackson I, Arafah BM, Nippoldt TB, Cook DM, Mooradian AD, Kwan A, Scism-Bacon J, Chipman JJ, Hartman ML. 2007. Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiency. J Bone Miner Res 22:762–770 [DOI] [PubMed] [Google Scholar]
- 35. Bex M, Abs R, Maiter D, Beckers A, Lamberigts G, Bouillon R. 2002. The effects of growth hormone replacement therapy on bone metabolism in adult-onset growth hormone deficiency: a 2-year open randomized controlled multicenter trial. J Bone Miner Res 17:1081–1094 [DOI] [PubMed] [Google Scholar]
- 36. Miller KK, Biller BM, Hier J, Arena E, Klibanski A. 2002. Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab 87:2770–2776 [DOI] [PubMed] [Google Scholar]
- 37. Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A. 2002. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 87:4177–4185 [DOI] [PubMed] [Google Scholar]
- 38. Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A. 1999. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84:2049–2055 [DOI] [PubMed] [Google Scholar]
- 39. Bredella MA, Misra M, Miller KK, Klibanski A, Gupta R. 2008. Trabecular structure analysis of the distal radius in adolescent patients with anorexia nervosa using ultra high resolution flat panel based volume CT. J Musculoskelet Neuronal Interact 8:315. [PubMed] [Google Scholar]
- 40. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. 2010. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone 46:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ. 2005. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565 [DOI] [PubMed] [Google Scholar]
- 42. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. 2003. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- 43. Halmi KA, Agras WS, Crow S, Mitchell J, Wilson GT, Bryson SW, Kraemer HC. 2005. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry 62:776–781 [DOI] [PubMed] [Google Scholar]