Abstract
Evaluation of the child with fractures is challenging, as no clear guidelines exist to distinguish traumatic from pathological fractures. Although most fractures in childhood are benign, recurrent fractures may be associated with a wide variety of primary skeletal diseases as well as secondary causes, necessitating a careful history and physical exam to guide the evaluation. There is no “gold standard” for the evaluation and treatment of children with fractures and low bone mineral density (BMD); therefore, the diagnosis of osteoporosis in a pediatric patient should be made using a combination of clinical and radiographic features. Interpretation of bone densitometry in growing patients presents a unique set of challenges because areal BMD measured by dual-energy x-ray absorptiometry depends on multiple dynamic variables. Interpretation of pediatric dual-energy x-ray absorptiometry should be based on Z-scores (sd scores compared to age, sex, and ethnicity-matched controls), using normative databases specific to the brand of densitometer and the patient population. Given the skeleton's ability to recover from low BMD through modeling and remodeling, optimizing management of underlying conditions leading to bone fragility is the initial step. Conservative measures including calcium and vitamin D supplementation and weight-bearing physical activity are important interventions that should not be overlooked. The use of bisphosphonates in children and adolescents is controversial due to lack of long-term efficacy and safety data and should be limited to clinical trials and compassionate therapy in children with significantly compromised quality of life. Close monitoring is required, and further study is necessary to assess their long-term safety and efficacy in children.
Accreditation and Credit Designation Statements.
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 CreditTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to:
Recognize the numerous primary and secondary causes of childhood osteoporosis
Perform a complete evaluation of a child with fractures, including the importance of a thorough history and physical exam
Consider confounding variables in the interpretation of DXA scans in pediatric patients, including body size, bone age and pubertal status
Select the most appropriate pharmacologic and non-pharmacologic therapies for the management of low bone density in children
Target Audience
This Journal-based CME activity should be of substantial interest to endocrinologists.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this CME activity are required to disclose to learners any relevant financial relationship(s) that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved or managed all identified conflicts of interest, as applicable.
Disclosures for JCEM Editors are found at http://www.endo-society.org/journals/Other/faculty_jcem.cfm.
The following individuals reported NO relevant financial relationships:
Alison Boyce, M.D., Rachel Gafni, M.D., and Leonard Wartofsky, M.D., reported no relevant financial relationships.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
This activity is not supported by grants, other funds, or in-kind contributions from commercial supporters.
Privacy and Confidentiality Statement
The Endocrine Society will record learner's personal information as provided on CME evaluations to allow for issuance and tracking of CME certificates. No individual performance data or any other personal information collected from evaluations will be shared with third parties.
Method of Participation
This Journal-based CME activity is available in print and online as full text HTML and as a PDF that can be viewed and/or printed using Adobe Acrobat Reader. To receive CME credit, participants should review the learning objectives and disclosure information; read the article and reflect on its content; then go to http://jcem.endojournals.org and find the article, click on CME for Readers, and follow the instructions to access and complete the post-activity test questions and evaluation. The estimated time to complete this activity, including review of material, is 1 hour. If you have questions about this CME activity, please direct them to education@endo-society.org.
Activity release date: July 2011
Activity expiration date: July 2012
Case
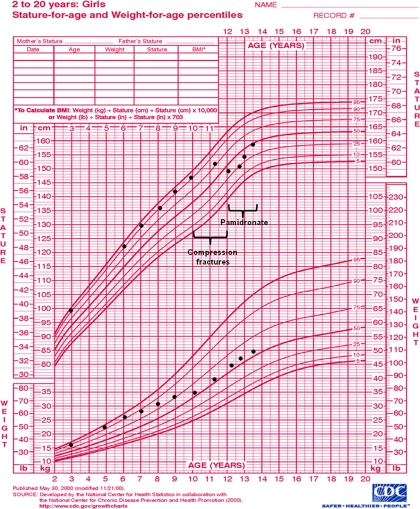
A 12-yr-old girl presents with a history of multiple vertebral compression fractures. At age 10, she fractured T6 while riding a roller coaster. She was evaluated by an orthopedist who instructed her to resume her regular activities, including lacrosse and basketball. At age 10 ¾, she fractured T3 playing sports, and at age 11 ½, she fractured T5 playing laser tag. Two additional thoracic vertebral fractures soon followed. She was seen by a hematologist who performed a bone marrow biopsy of T6, which was normal. She is an otherwise healthy girl, previously very athletic, with normal puberty. Her only complaint is back pain, treated with pain medications and muscle relaxants. She consumes at least three servings of dairy per day. Growth had been normal along the 95th percentile until the onset of the vertebral fractures, causing height loss such that by age 12 she was at the 25th percentile (Fig. 1). The patient's father is 183 cm tall and reports a history of several sports-related fractures as a child; he has normal hearing and sclerae. Her mother is 170 cm tall; she and the patient's siblings are healthy. The physical exam reveals a bright, nondysmorphic girl with an arm span 5 cm longer than her height, mildly translucent teeth, normal sclerae, kyphoscoliosis, and decreased flexibility. Hearing and vision are normal. A dual-energy x-ray absorptiometry (DXA) scan was performed and revealed a decreased lumbar spine Z-score of −3.9.
Fig. 1.
Growth chart of girl with osteoporosis. Decline in height percentile with absolute height loss is apparent after the development of multiple thoracic compression fractures between ages 10 and 12 yr. Pamidronate was initiated at age 12, associated with recovery of height and continued linear growth.
Background
Rising awareness of osteoporosis has led pediatric practitioners to screen for and consider treatment of low bone mineral density (BMD) in children with fractures. Although standards for diagnosis and treatment of osteoporosis exist in adults, differences in the etiology and clinical significance of bone fragility in children make extrapolation of adult data inappropriate. Lack of data in children and adolescents has prevented the development of a “gold-standard” when approaching the child with fractures.
Fractures in Childhood
Fractures are common in the pediatric population, with an incidence of approximately 50% in boys and 40% in girls (1–3). Fracture rate appears to be increasing over time (1–5), particularly at the distal radius, which remains the most common site of fracture in children and adolescents. Fracture rate peaks between ages 11 and 15 yr (2, 4–7), corresponding to the period of maximum postnatal growth velocity. There is relative undermineralization of the adolescent skeleton, due to a delay of approximately 8 months between the period of peak growth velocity and peak bone mineral accrual (8, 9). This leads to transient cortical weakness, particularly of the distal radius in adolescent boys (10), which may contribute to the increased incidence of metaphyseal forearm fractures. Increased participation in competitive youth sports has also led to a concurrent rise in pediatric overuse injuries, such as stress fractures (11).
Distinguishing a traumatic from pathological fracture is often difficult because the literature has not clearly defined what constitutes a fragility fracture. Vertebral compression fractures and femur fractures in the absence of a significant trauma, such as a motor vehicle accident or fall from a great height, are rare in childhood and should be considered pathological fractures. In some cases a child may present with a more minor fracture and no reported history of trauma. In such situations, it can be difficult to determine whether this is a true atraumatic fracture or a case of an inaccurate history. Parents may report a history of multiple fractures treated with short-term immobilization; however, review of the radiographs may reveal that some of these injuries were sprains treated with splinting. One must always consider the possibility of physical abuse in a child who presents with repeated injuries, fractures in infants, and fractures that appear inconsistent with the reported history.
Differential Diagnosis
The list of conditions associated with an increased risk of fragility fractures in childhood is long (Table 1). However, many of these conditions are quickly diagnosed or excluded with a thorough history and physical exam along with selected diagnostic tests. Primary bone disorders leading to juvenile osteoporosis are relatively rare (Table 1). The most common cause of primary osteoporosis is osteogenesis imperfecta (OI), a syndrome of increased bone fragility due to defects in the quality or quantity of collagen I. There are several subtypes, ranging from mild to perinatal lethal. Type I OI is the most common and mildest form, typically presenting in early adolescence with clinical improvement after puberty. On examination, these children are often of normal stature before fracture and may have blue-tinted sclerae, dental involvement (dentinogenesis imperfecta), and an affected parent; hearing loss develops in approximately 50% of affected individuals by adulthood (12). Genetic testing for abnormalities in the genes encoding collagen I (COL1A1/COL1A2) is commercially available and identifies over 90% of patients with OI (13). Analysis of type I collagen synthesized in vitro by culturing dermal fibroblasts from skin biopsy has an estimated sensitivity of 87% in nonlethal OI and 98% in the lethal form (14). Because negative testing cannot definitively exclude OI, practitioners must rely on clinical characteristics to determine diagnosis and treatment. In patients with negative genetic testing, normal sclerae and hearing, and absent family history, one must consider idiopathic juvenile osteoporosis (IJO), a rare condition characterized by multiple fragility fractures, which also typically arises in the school-age child and spontaneously remits after puberty. Similar to type I OI, the overall prognosis for IJO is favorable, although some patients with severe disease may have permanent disabling deformities of the spine and long bones (15). The diagnosis of IJO can be, made only after likely secondary causes have been excluded. Primary juvenile osteoporosis can also be seen in connective tissue disorders and other rare genetic conditions associated with intrinsic skeletal defects, including the osteoporosis pseudoglioma syndrome, an autosomal recessive disorder due to inactivating mutations in low-density lipoprotein receptor-related protein 5 (LRP5). This diagnosis should be considered in any patient with visual impairment. Heterozygous mutations in LRP5 have also been reported in autosomal dominant forms of juvenile osteoporosis (16). Fragility fractures can also occur despite high BMD in sclerosing bone disorders due to impaired osteoclast function, such as pycnodysostosis and osteopetrosis.
Table 1.
Disorders associated with fragility fractures in children (not all-inclusive)
| Primary conditions |
| Genetic disorders (selected) |
| Osteogenesis imperfecta |
| Osteoporosis pseudoglioma syndrome |
| Ehlers-Danlos syndrome |
| Marfan syndrome |
| Homocystinuria |
| Hajdu-Cheney Syndrome |
| Pycnodysostosis |
| Osteopetrosis |
| Hypophosphatasia |
| Polyostotic fibrous dysplasia |
| Rickets (genetic forms) |
| Idiopathic juvenile osteoporosis |
| Secondary conditions |
| Chronic inflammatory conditions |
| Systemic lupus erythematosis |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Reduced mobility |
| Cerebral palsy |
| Duchenne muscular dystrophy |
| Posttraumatic |
| Infiltrative |
| Leukemia |
| Thalassemia |
| Mastocytosis |
| Endocrine |
| Hypogonadism |
| GH deficiency |
| Cushing syndrome |
| Hyperthyroidism |
| Diabetes mellitus |
| Female athlete triad |
| Nutritional/malabsorptive |
| Vitamin D deficiency |
| Celiac disease |
| Biliary atresia |
| Cystic fibrosis |
| Anorexia nervosa |
| Renal |
| Chronic kidney disease |
| Secondary hyperparathyroidism |
| Iatrogenic |
| Glucocorticoids |
| Anticonvulsants |
| Methotrexate |
| Radiation therapy |
| Antiretrovirals |
Secondary osteoporosis is observed as a complication of many chronic conditions, particularly those associated with inflammatory disease, malabsorption, endocrine/metabolic disturbances, decreased mobility or prolonged glucocorticoid therapy (Table 1). In these conditions, low BMD may be a sequela of the primary disorder, a complication of treatment, or a combination. Vitamin D deficiency and decreased dietary calcium intake can contribute to low BMD, in addition to causing rickets. Decreased BMD has also been reported in children with idiopathic hypercalciuria (17).
Evaluation
Bone densitometry
Any child with a history of fragility fractures should be assessed with bone densitometry. A significant family history of recurrent fractures is suggestive of an underlying inherited condition, and in these cases it is often helpful to study first-degree family members. An expert panel has recently recommended densitometry for children with a history of clinically significant fractures, defined as a long bone fracture of the lower extremities, a vertebral compression fracture, or two or more long-bone fractures of the upper extremities (18). The decision to screen becomes less clear when evaluating children with multiple trauma-related fractures. For most patients, assessment is made on an individual basis, taking into consideration severity and number of fractures, along with other risk factors.
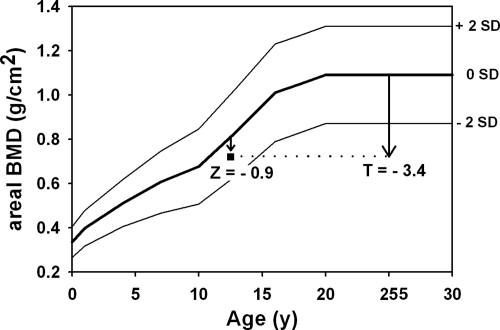
Multiple methods are available for assessing bone density including DXA, quantitative computed tomography (QCT), and quantitative ultrasound. Plain radiographs are not recommended for quantification of BMD but are useful for identifying fractures, deformity, skeletal dysplasias, rickets, and sclerosing bone disorders. DXA is currently the preferred method, given its wide availability, rapid scanning time, and low radiation. The sites typically studied are the postero-anterior lumbar spine, proximal femur, distal radius, and total body. The postero-anterior spine and TBLH (total body without the head) for evaluation and monitoring because they are the most reproducible and accurate sites measured by DXA (19, 20). In children with impaired mobility, scans of the distal femur may be more informative (21). A comprehensive pediatric reference database for Hologic densitometers is available (22); it is important that only normative databases specific to the brand of densitometer be used for interpretation. Z-scores should be calculated as sd scores compared with age-, sex-, and ethnicity-matched controls. The diagnosis of low BMD in a child should never be made on the basis of T-score (sd score compared with young adults at peak bone mass) (Fig. 2). This error has led to the overdiagnosis of low BMD in children (23).
Fig. 2.
BMD Z-score and T-score for a 12-yr-old child (23). The curves demarcate the normal (mean ± 2 sd) areal BMD of the lumbar spine, which depends on skeletal size and thus increases with age. The square symbol represents a patient who received an erroneous diagnosis of osteoporosis based on a low T-score. The Z-score of this patient is well within the normal range for age.
DXA is a two-dimensional technology measuring areal BMD, calculated as the bone mineral content divided by the area of interest, reported in grams per square centimeter. The inability of DXA to measure the true three-dimensional volume of the bone has important implications because areal BMD will be decreased in smaller bones and increased in larger bones. A patient with short stature may therefore have abnormally low areal BMD compared with the average population despite normal true volumetric BMD (24). There are several methods that may be applied to correct areal BMD for body size, using mathematical models (25, 26) and adjusting TBLH or body composition (27). Interpretation of DXA scans should include consideration of not only height but also bone age and pubertal status. Lean body mass has been shown to correlate with BMD (28–30), which may have particular significance in patients with complications of chronic disease leading to relative sarcopenia. Methods have been developed to estimate the effects of lean body mass on BMD (29, 30); however, these adjustments may be problematic in children with fluctuating body composition, acute exacerbations of underlying disease, and/or their treatment. In addition, local structural changes such as vertebral fractures, osteophytes, and arterial calcifications can lead to spuriously elevated BMD measurements (31).
Quantitative ultrasound can be used to assess BMD; however, diagnostic significance has not been well-established, and it is not recommended. QCT of the spine has the advantage over DXA in that it can distinguish between trabecular and cortical bone and can determine true three-dimensional volumetric BMD (32). Unfortunately, expense, availability, and high radiation exposure makes axial QCT impractical for routine clinical use. QCT can be performed on the peripheral skeleton using much less radiation; however, this technique is confounded by the continually changing size and shape of the growing skeleton (33). There are multiple emerging modalities, used primarily in research, such as high-resolution peripheral QCT, high-resolution magnetic resonance imaging, and micro-computed tomography, which surmount many of the limitations of DXA and have the potential for future clinical use.
The clinical definition of osteoporosis includes low BMD, increased bone fragility with associated changes in bony microarchitecture, and an increased risk of fracture (34). There is evidence in adults that low BMD is predictive of increased fracture risk, and a BMD T-score less than −2.5 sd is suggestive of osteoporosis in adults (35). The clinical relevance of low BMD in children has not been well-established, although there are retrospective data that suggest an increased fracture risk when the Z-score is less than −2 (36) and one prospective study reporting an 89% increase in fracture risk for every sd decrease in size-adjusted BMD (27). However, spine BMD by DXA may overlook clinically apparent osteoporosis, as was recently described in a child with multiple thoracic compression fractures despite a normal lumbar spine Z-score (37). Concurrent vertebral fracture assessment done by DXA has lower radiation than plain radiographs and is useful in adults; unfortunately, the current software is inadequate for pediatric use (38). The diagnosis and management of osteoporosis in the pediatric patient must therefore be based on a combination of clinical and radiographic findings, rather than relying upon bone densitometry alone. The International Society for Clinical Densitometry recently updated its official position on DXA evaluation in children and adolescents, recommending that the “diagnosis of osteoporosis requires the presence of both a clinically significant fracture history and low bone mineral content or bone mineral density” (20, 39).
Conventional densitometry methods provide information about bone mass. However, bone strength is dependent not only on bone mass and density but also the structural properties that resist bending and fracture. Mathematical models exist that use DXA and QCT to estimate measurements of bone strength (40, 41). Further research into this area may yield applications in the clinical setting.
Identification of underlying etiology
Given the extensive list of possible causes for fracture with low bone density, testing must be guided by the history and physical exam. At a minimum, we recommend evaluation of routine hematologic and biochemical indices, erythrocyte sedimentation rate, intact PTH, serum calcium and phosphorus, urinary calcium excretion, and screening for celiac disease. Patients should be screened for vitamin D deficiency using a serum 25-hydroxyvitamin D level. Bone marrow aspirates, endoscopy/colonoscopy, liver biopsy, and genetic tests may be performed where indicated but are not done routinely in an otherwise healthy, asymptomatic child. Given the clinical similarities between type I OI and IJO, we recommend genetic testing of COL1A1/COL1A2 for all children before assigning a diagnosis of IJO. In IJO, additional search for mutations in genes related to osteoblast and osteoclast recruitment and differentiation, as well as mutations in LRP5 may lead to a better understanding of the genetic determinants of bone density. Finally, measurement of bone turnover markers such as bone-specific alkaline phosphatase, osteocalcin, collagen cross-linked N-telopeptide, etc., may suggest a “low-turnover” or “high-turnover” state that can help guide therapy. Results must be interpreted with caution because these markers vary relative to age and skeletal maturation, and pediatric normative data are limited (42). Additionally, elevated markers in the setting of acute fracture may be misleading.
Bone biopsy
When a diagnosis is unclear, the definitive method for assessment of bone density, turnover, and microarchitecture is the bicortical transiliac bone biopsy. Administration of tetracycline before biopsy, which is incorporated into areas of active bone turnover, allows for measurement of dynamic indices such as bone formation rate and mineral apposition rate. This procedure is particularly helpful for distinguishing low-turnover from high-turnover osteoporosis and identifying osteomalacia. Thus, this procedure is most often considered in a child with severe osteoporosis of unknown etiology because it may provide diagnostic clues and direct therapeutic intervention. For example, a biopsy demonstrating a paucity of osteoclasts suggests that bisphosphonate therapy may be ineffective. However, given its invasiveness, bone biopsy is rarely performed in children.
Treatment
The first step is to identify and treat underlying causes of low BMD and use bone-sparing therapies whenever possible. Studies in juvenile animals and children have demonstrated that deceased BMD during childhood is largely reversible with remission or optimized management of the underlying disease process (43–45).
In healthy children with multiple traumatic fractures and normal densitometry, observation is usually the best course, with repeat densitometry performed on an as-needed basis. Adequate rest and rehabilitation are essential in patients with stress fractures to prevent recurrence and complications (46). Ensuring adequate calcium intake and vitamin D stores is important for all patients, recognizing that the recommended daily intakes may not be sufficient for patients with primary bone disorders, malabsorption, or other chronic conditions. The Institute of Medicine recently released recommendations stating that serum 25-hydroxyvitamin D levels should be maintained above 20 ng/ml (50 nmol/liter) to optimize bone health (47). Weight-bearing physical activity is critical for bone health, and children with reduced mobility have been shown to gain bone mass with physical therapy (48) and standing on vibrating platforms (49). It is important to counsel the family and the school regarding activities that may predispose a child with osteoporosis to further fractures. Typical restrictions include the avoidance of jarring activities (e.g. horseback riding, roller coasters), contact sports, forward flexion exercises, and heavy backpacks. Children often require a separate set of schoolbooks at home to avoid excessive carrying.
In addition to calcium and vitamin D, there are several Food and Drug Administration (FDA)-approved therapies to treat osteoporosis in adults but no FDA-approved therapies for children. Calcitonin has not demonstrated significant decreases in adult fractures and is rarely used. Teriparatide (PTH 1–34) is a very effective anabolic agent; however, its black box warning regarding the risk of osteosarcoma precludes its use in children except in perhaps extreme circumstances. Denosumab, a recently approved RANKL (receptor activator of nuclear factor-κB ligand inhibitor), is a potent antiresorptive agent; pediatric trials have yet to be conducted. Bisphosphonates are approved for adults with osteoporosis, Paget's disease, and hypercalcemia. They are not indicated in sclerosing bone diseases (e.g. osteopetrosis) or primary mineralization disorders (e.g. hypophosphatasia or rickets). Although there are numerous publications reporting bisphosphonate use in pediatric populations, few have adequate controls or long-term safety data. The greatest experience comes from use in patients with OI, where bisphosphonates have been found to improve BMD, reduce functional impairment, and relieve pain (50–52). Bisphosphonates are now widely used for treatment of this disorder, although uncertainty remains regarding appropriate regimen, method, and dose. Bisphosphonates also may be effective in other forms of primary osteoporosis including IJO (53) and the osteoporosis pseudoglioma syndrome (54), although definitive recommendations cannot be made due to the lack of controlled data (55).
The use of bisphosphonates for treatment of secondary osteoporosis in children is also unclear. Gains in BMD have been demonstrated in several pediatric populations, including children with cerebral palsy (56), postrenal transplant (57), and rheumatologic conditions (58). A recent Cochrane review examining bisphosphonate use in children with secondary osteoporosis concluded that data are not currently sufficient to support use of bisphosphonates as standard therapy (59). Short-term data over a 3-yr period found the medications to be well-tolerated, and preliminary positive effects on BMD and pain were sufficient to justify compassionate use for patients with significantly impacted quality of life.
Pediatric dosing of bisphosphonates has not been established, as evidenced by the wide variability of dosing regimens of the published studies (60). Practitioners may be inclined to treat all causes of juvenile osteoporosis with the OI pamidronate protocols of 0.5–1 mg/kg dose on 3 consecutive days every 2–4 months. However, small studies suggest that improvements in BMD can be achieved with a single infusion every 3–6 months (61–63). Newer bisphosphonates have been approved for use in postmenopausal osteoporosis as an iv push given every 3 months (ibandronate) and a once-yearly infusion (zoledronate). Although administration of these drugs is much easier than pamidronate and could be considered for older adolescents, there are no pediatric data supporting efficacy and safety of these regimens. For oral agents, there is even less consensus as to the correct dose; some studies use an individualized milligrams per kilogram dose, whereas others use a weight cutoff to divide subjects into two dosing groups (60). Whatever dose is ultimately chosen, monitoring for efficacy and side effects is important.
Side Effects and Monitoring of Bisphosphonates
Bisphosphonates act through inhibition of osteoclastic bone resorption, placing patients at risk for hypocalcemia. Vitamin D stores must be adequate before initiating treatment, and calcium and vitamin D intake should be optimized for the duration of therapy. Many children experience an acute phase reaction with the initial dose of oral or iv bisphosphonate, which can include fever, malaise, diarrhea, nausea, and bone or muscle pain. Symptoms typically begin within the first 24 h of treatment and last 1–3 d. Starting with a lower initial dose and premedicating with nonsteroidal antiinflammatory medications can potentially decrease the severity of these symptoms.
In adults, bisphosphonates have been associated, often without direct evidence of causality, with a number of side effects including musculoskeletal pain, irritant esophagitis and esophageal cancer, atrial fibrillation, and iritis (64). Given the risk of gastrointestinal ulcerations associated with the oral forms, administration instructions should be reviewed at every visit, and the patients should be questioned for any gastrointestinal complaints. We do not recommend the use of oral bisphosphonates in patients with gastroesophageal disease, significant neuromuscular disease, or impaired cognition because they may be unable to report epigastric pain. Osteonecrosis of the jaw has been linked to use of bisphosphonates; however, this complication is typically seen in the setting of multiple high-dose iv infusions in adult cancer patients, and there have been no reported cases in children or adolescents (65). Nonetheless, we recommend a full dental exam and the completion of any required dental work before initiating treatment, along with maintenance of good oral hygiene. Bisphosphonates may negatively affect orthodontic treatment in adults (66); the effects in children are unknown.
Bisphosphonates have a long skeletal half-life and can be detected in the urine of children years after discontinuation (67), raising concerns surrounding release from bone during pregnancy. Bisphosphonates cross the placenta easily, and administration of high doses in pregnant rats was found to be associated with fetal skeletal anomalies (68). A recent prospective study of 21 women exposed to bisphosphonates during or within the first 3 months before pregnancy found no difference in major birth defects compared with controls (69). Although preliminary data in humans are encouraging, families must be informed that the risk to the fetus is unknown. Practitioners should screen for pregnancy before initiating therapy and should counsel sexually active girls on effective birth control for the duration of treatment.
Long-term suppression of bone modeling and remodeling may have important repercussions in a growing skeleton. Reports suggest that linear growth continues normally with bisphosphonate therapy in IJO (53); however, abnormalities in modeling at the distal femur and radius have been demonstrated in OI even after discontinuation of bisphosphonate therapy (70). A child treated with excessive doses of pamidronate developed osteopetrosis with persistent remodeling defects more than 6 yr after discontinuation (71, 72). The long-term effects on fracture healing and bone strength have yet to be elucidated. One study in patients with OI reported delayed fracture healing despite gains in BMD (73). Another study found no effect on healing of spontaneous fractures, but delayed healing after osteotomies (74); thus, we often recommend discontinuation of bisphosphonates several months before scheduled orthopedic procedures. Reports of atypical fractures in adults treated with long-term bisphosphonates further raises questions regarding the safety of continuous suppression of bone remodeling (75). These concerns have led to the suggestion that bisphosphonate therapy be interrupted every few years, depending upon the severity of the bone disease (64, 75).
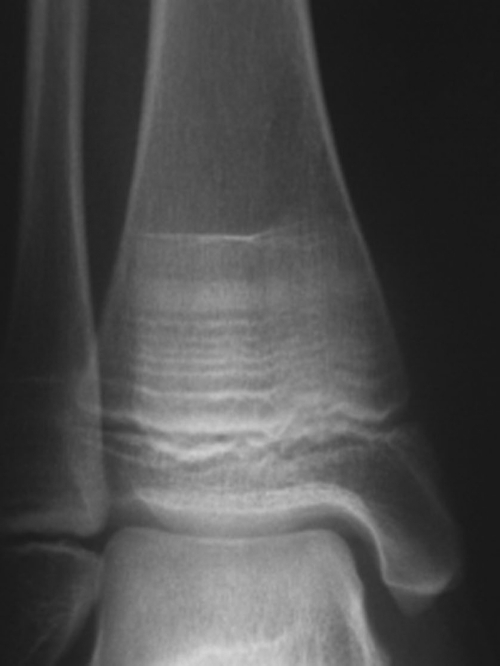
Patients on bisphosphonate therapy should undergo follow-up densitometry to monitor treatment; the interval between studies depends upon the severity of disease, but most agree that studies should not be done more frequently than every 6 months (39). Ideally, these studies should be performed at the same center to facilitate comparison with previous exams. Discontinuation of bisphosphonates should be considered when the Z-score is greater than −2 and/or the patient is no longer fracturing. Children with open epiphyses should have periodic radiographs of long bones to monitor for modeling defects. Typical changes include hyperdense, sclerotic bands representing areas of decreased bone turnover and slight widening of distal metaphyses resulting from mild undertubulation (76, 77) (Fig. 3). These findings tend to resolve after discontinuation of therapy and closure of epiphyses. Intermittent measurements of bone turnover markers are a noninvasive measure of bone activity and may provide additional information regarding response to therapy.
Fig. 3.
Hyperdense sclerotic bands in the epiphyses of a child treated with serial infusions of pamidronate. [Reproduced with permission from M. Al Muderis et al.: J Bone Joint Surg Am 89:1511–1516, 2007 (76).]
Case Follow-up
Our 12-yr-old patient underwent a battery of tests including screening for rheumatologic disease, celiac disease, thyroid dysfunction, hypercalciuria, and vitamin D deficiency, all of which were normal. Markers of bone turnover were unremarkable, and no mutations in COL1A1/COL1A2 were detected. The patient was given a presumptive diagnosis of IJO and started on vitamin D supplementation, physical therapy, weight-bearing exercise, and pamidronate infusions every 3 months. She was restricted from playing contact sports. Over the course of 18 months, she experienced a decrease in back pain, and an increase in height achieving the 50th percentile for age (Fig 1). She did not experience any fractures during that time. A DXA scan after 12 months of therapy revealed an improved spine Z-score of −2.5.
Conclusion
Fragility fractures in children may be due to a wide variety of genetic, medical, or nutritional disorders. The decision to perform screening densitometry in a child or adolescent must be made on an individual basis, taking into account fracture history and risk factors. The clinical implications of low BMD in the pediatric population have not been well-established, and the diagnosis of osteoporosis must be made in association with clinical history rather than relying upon bone densitometry alone. The primary step in treatment should include management of underlying conditions as well as conservative measures including vitamin D and calcium supplementation and weight-bearing physical activity. The use of bisphosphonates in children and adolescents is controversial due to lack of long-term efficacy and safety data and should be limited to clinical trials and as compassionate therapy in children with significantly compromised quality of life.
Supplementary Material
Acknowledgments
This manuscript reflects the opinions of the authors and not those of the National Institutes of Health or the U.S. Federal Government.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMD
- Bone mineral density
- DXA
- dual-energy x-ray absorptiometry
- IJO
- idiopathic juvenile osteoporosis
- LRP5
- low-density lipoprotein receptor-related protein 5
- OI
- osteogenesis imperfecta
- QCT
- quantitative computed tomography.
References
- 1. Landin LA. 1997. Epidemiology of children's fractures. J Pediatr Orthop B 6:79–83 [DOI] [PubMed] [Google Scholar]
- 2. Hedström EM, Svensson O, Bergström U, Michno P. 2010. Epidemiology of fractures in children and adolescents. Acta Orthop 81:148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones IE, Williams SM, Dow N, Goulding A. 2002. How many children remain fracture-free during growth? a longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int 13:990–995 [DOI] [PubMed] [Google Scholar]
- 4. Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. 2003. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA 290:1479–1485 [DOI] [PubMed] [Google Scholar]
- 5. Mäyränpää MK, Mäkitie O, Kallio PE. 2010. Decreasing incidence and changing pattern of childhood fractures: a population-based study. J Bone Miner Res 25:2476–2483 [DOI] [PubMed] [Google Scholar]
- 6. Landin LA. 1983. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl 202:1–109 [PubMed] [Google Scholar]
- 7. Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, Barker D. 1995. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res 10:940–947 [DOI] [PubMed] [Google Scholar]
- 8. Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. 2000. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res 15:2245–2250 [DOI] [PubMed] [Google Scholar]
- 9. Fournier PE, Rizzoli R, Slosman DO, Theintz G, Bonjour JP. 1997. Asynchrony between the rates of standing height gain and bone mass accumulation during puberty. Osteoporos Int 7:525–532 [DOI] [PubMed] [Google Scholar]
- 10. Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. 2010. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res 25:1521–1526 [DOI] [PubMed] [Google Scholar]
- 11. Heyworth BE, Green DW. 2008. Lower extremity stress fractures in pediatric and adolescent athletes. Curr Opin Pediatr 20:58–61 [DOI] [PubMed] [Google Scholar]
- 12. Basel D, Steiner RD. 2009. Osteogenesis imperfecta: recent findings shed new light on this once well-understood condition. Genet Med 11:375–385 [DOI] [PubMed] [Google Scholar]
- 13. Sykes B, Ogilvie D, Wordsworth P, Wallis G, Mathew C, Beighton P, Nicholls A, Pope FM, Thompson E, Tsipouras P, Schwartz R, Jensson O, Arnason A, Børresen A, Heiberg A, Frey D, Steinmann B. 1990. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet 46:293–307 [PMC free article] [PubMed] [Google Scholar]
- 14. Wenstrup RJ, Willing MC, Starman BJ, Byers PH. 1990. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet 46:975–982 [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenc RS. 2002. Idiopathic juvenile osteoporosis. Calcif Tissue Int 70:395–397 [DOI] [PubMed] [Google Scholar]
- 16. Hartikka H, Mäkitie O, Männikkö M, Doria AS, Daneman A, Cole WG, Ala-Kokko L, Sochett EB. 2005. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res 20:783–789 [DOI] [PubMed] [Google Scholar]
- 17. Zerwekh JE. 2010. Bone disease and hypercalciuria in children. Pediatr Nephrol 25:395–401 [DOI] [PubMed] [Google Scholar]
- 18. Rauch F, Plotkin H, DiMeglio L, Engelbert RH, Henderson RC, Munns C, Wenkert D, Zeitler P. 2008. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2007 Pediatric Official Positions. J Clin Densitom 11:22–28 [DOI] [PubMed] [Google Scholar]
- 19. Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. 2008. Dual energy x-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]
- 20. Bishop N, Braillon P, Burnham J, Cimaz R, Davies J, Fewtrell M, Hogler W, Kennedy K, Mäkitie O, Mughal Z, Shaw N, Vogiatzi M, Ward K, Bianchi ML. 2008. Dual-energy x-ray absorptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:29–42 [DOI] [PubMed] [Google Scholar]
- 21. Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, Henderson RC. 2009. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy x-ray absorptiometry. J Clin Densitom 12:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. 2007. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]
- 23. Gafni RI, Baron J. 2004. Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy x-ray absorptiometry (DEXA). J Pediatr 144:253–257 [DOI] [PubMed] [Google Scholar]
- 24. Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. 2005. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr 146:776–779 [DOI] [PubMed] [Google Scholar]
- 25. Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. 2010. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. 1999. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 84:4702–4712 [DOI] [PubMed] [Google Scholar]
- 27. Clark EM, Ness AR, Bishop NJ, Tobias JH. 2006. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 21:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogle GD, Allen JR, Humphries IR, Lu PW, Briody JN, Morley K, Howman-Giles R, Cowell CT. 1995. Body-composition assessment by dual-energy x-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr 61:746–753 [DOI] [PubMed] [Google Scholar]
- 29. Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. 2004. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 35:965–972 [DOI] [PubMed] [Google Scholar]
- 30. Högler W, Briody J, Woodhead HJ, Chan A, Cowell CT. 2003. Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J Pediatr 143:81–88 [DOI] [PubMed] [Google Scholar]
- 31. Rand T, Seidl G, Kainberger F, Resch A, Hittmair K, Schneider B, Glüer CC, Imhof H. 1997. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy x-ray absorptiometry (DXA). Calcif Tissue Int 60:430–433 [DOI] [PubMed] [Google Scholar]
- 32. Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. 2005. Bone acquisition in healthy children and adolescents: comparisons of dual-energy x-ray absorptiometry and computed tomography measures. J Clin Endocrinol Metab 90:1925–1928 [DOI] [PubMed] [Google Scholar]
- 33. Lee DC, Gilsanz V, Wren TA. 2007. Limitations of peripheral quantitative computed tomography metaphyseal bone density measurements. J Clin Endocrinol Metab 92:4248–4253 [DOI] [PubMed] [Google Scholar]
- 34. 2000. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement 17:1–45 [PubMed] [Google Scholar]
- 35. Kanis JA. 2002. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936 [DOI] [PubMed] [Google Scholar]
- 36. Jones G, Ma D, Cameron F. 2006. Bone density interpretation and relevance in Caucasian children aged 9–17 years of age: insights from a population-based fracture study. J Clin Densitom 9:202–209 [DOI] [PubMed] [Google Scholar]
- 37. Sbrocchi AM, Rauch F, Matzinger M, Feber J, Ward LM. 2011. Vertebral fractures despite normal spine bone mineral density in a boy with nephrotic syndrome. Pediatr Nephrol 26:139–142 [DOI] [PubMed] [Google Scholar]
- 38. Mäyränpää MK, Helenius I, Valta H, Mäyränpää MI, Toiviainen-Salo S, Mäkitie O. 2007. Bone densitometry in the diagnosis of vertebral fractures in children: accuracy of vertebral fracture assessment. Bone 41:353–359 [DOI] [PubMed] [Google Scholar]
- 39. Bianchi ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, Leonard MB, Kalkwarf HJ. 2010. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatr Nephrol 25:37–47 [DOI] [PubMed] [Google Scholar]
- 40. Gatti D, Sartori E, Braga V, Corallo F, Rossini M, Adami S. 2001. Radial bending breaking resistance derived by densitometric evaluation predicts femoral neck fracture. Osteoporos Int 12:864–869 [DOI] [PubMed] [Google Scholar]
- 41. Schoenau E, Neu CM, Rauch F, Manz F. 2001. The development of bone strength at the proximal radius during childhood and adolescence. J Clin Endocrinol Metab 86:613–618 [DOI] [PubMed] [Google Scholar]
- 42. Szulc P, Seeman E, Delmas PD. 2000. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int 11:281–294 [DOI] [PubMed] [Google Scholar]
- 43. García-De Alvaro MT, Muñoz-Calvo MT, Martínez G, Barrios V, Hawkins F, Argente J. 2007. Regional skeletal bone deficit in female adolescents with anorexia nervosa: influence of the degree of malnutrition and weight recovery in a two year longitudinal study. J Pediatr Endocrinol Metab 20:1223–1231 [DOI] [PubMed] [Google Scholar]
- 44. Mora S, Barera G, Ricotti A, Weber G, Bianchi C, Chiumello G. 1998. Reversal of low bone density with a gluten-free diet in children and adolescents with celiac disease. Am J Clin Nutr 67:477–481 [DOI] [PubMed] [Google Scholar]
- 45. Gafni RI, McCarthy EF, Hatcher T, Meyers JL, Inoue N, Reddy C, Weise M, Barnes KM, Abad V, Baron J. 2002. Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J 16:736–738 [DOI] [PubMed] [Google Scholar]
- 46. Patel DR. 2010. Stress fractures: diagnosis and management in the primary care setting. Pediatr Clin North Am 57:819–827 [DOI] [PubMed] [Google Scholar]
- 47. 2010. Dietary reference intakes for calcium and vitamin D. Washington, DC: Institute of Medicine of the National Academies [Google Scholar]
- 48. Chad KE, Bailey DA, McKay HA, Zello GA, Snyder RE. 1999. The effect of a weight-bearing physical activity program on bone mineral content and estimated volumetric density in children with spastic cerebral palsy. J Pediatr 135:115–117 [DOI] [PubMed] [Google Scholar]
- 49. Caulton JM, Ward KA, Alsop CW, Dunn G, Adams JE, Mughal MZ. 2004. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child 89:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rauch F, Plotkin H, Zeitlin L, Glorieux FH. 2003. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res 18:610–614 [DOI] [PubMed] [Google Scholar]
- 51. DiMeglio LA, Peacock M. 2006. Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res 21:132–140 [DOI] [PubMed] [Google Scholar]
- 52. Löwing K, Aström E, Oscarsson KA, Söderhäll S, Eliasson AC. 2007. Effect of intravenous pamidronate therapy on everyday activities in children with osteogenesis imperfecta. Acta Paediatr 96:1180–1183 [DOI] [PubMed] [Google Scholar]
- 53. Brumsen C, Hamdy NA, Papapoulos SE. 1997. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine (Baltimore) 76:266–283 [DOI] [PubMed] [Google Scholar]
- 54. Streeten EA, McBride D, Puffenberger E, Hoffman ME, Pollin TI, Donnelly P, Sack P, Morton H. 2008. Osteoporosis-pseudoglioma syndrome: description of 9 new cases and beneficial response to bisphosphonates. Bone 43:584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Speiser PW, Clarson CL, Eugster EA, Kemp SF, Radovick S, Rogol AD, Wilson TA, Pharmacy L, Therapeutic C. 2005. Bisphosphonate treatment of pediatric bone disease. Pediatr Endocrinol Rev 3:87–96 [PubMed] [Google Scholar]
- 56. Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. 2002. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr 141:644–651 [DOI] [PubMed] [Google Scholar]
- 57. El-Husseini AA, El-Agroudy AE, El-Sayed MF, Sobh MA, Ghoneim MA. 2004. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatr Transplant 8:357–361 [DOI] [PubMed] [Google Scholar]
- 58. Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. 2005. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford) 44:813–818 [DOI] [PubMed] [Google Scholar]
- 59. Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, Rauch F, Tugwell P, Moher D. 2007. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev:CD005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bachrach LK, Ward LM. 2009. Clinical review 1: bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab 94:400–409 [DOI] [PubMed] [Google Scholar]
- 61. Gandrud LM, Cheung JC, Daniels MW, Bachrach LK. 2003. Low-dose intravenous pamidronate reduces fractures in childhood osteoporosis. J Pediatr Endocrinol Metab 16:887–892 [DOI] [PubMed] [Google Scholar]
- 62. González E, Pavía C, Ros J, Villaronga M, Valls C, Escolá J. 2001. Efficacy of low dose schedule pamidronate infusion in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab 14:529–533 [DOI] [PubMed] [Google Scholar]
- 63. Steelman J, Zeitler P. 2003. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J Pediatr 142:417–423 [DOI] [PubMed] [Google Scholar]
- 64. Watts NB, Diab DL. 2010. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 95:1555–1565 [DOI] [PubMed] [Google Scholar]
- 65. Chahine C, Cheung MS, Head TW, Schwartz S, Glorieux FH, Rauch F. 2008. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J Pediatr 153:719–720 [DOI] [PubMed] [Google Scholar]
- 66. Ghoneima AA, Allam ES, Zunt SL, Windsor LJ. 2010. Bisphosphonates treatment and orthodontic considerations. Orthod Craniofac Res 13:1–10 [DOI] [PubMed] [Google Scholar]
- 67. Papapoulos SE, Cremers SC. 2007. Prolonged bisphosphonate release after treatment in children. N Engl J Med 356:1075–1076 [DOI] [PubMed] [Google Scholar]
- 68. Patlas N, Golomb G, Yaffe P, Pinto T, Breuer E, Ornoy A. 1999. Transplacental effects of bisphosphonates on fetal skeletal ossification and mineralization in rats. Teratology 60:68–73 [DOI] [PubMed] [Google Scholar]
- 69. Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, Koren G, Ito S. 2009. Pregnancy outcome following in utero exposure to bisphosphonates. Bone 44:428–430 [DOI] [PubMed] [Google Scholar]
- 70. Rauch F, Cornibert S, Cheung M, Glorieux FH. 2007. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone 40:821–827 [DOI] [PubMed] [Google Scholar]
- 71. Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. 2008. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res 23:1698–1707 [DOI] [PubMed] [Google Scholar]
- 72. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. 2003. Bisphosphonate-induced osteopetrosis. N Engl J Med 349:457–463 [DOI] [PubMed] [Google Scholar]
- 73. Alharbi M, Pinto G, Finidori G, Souberbielle JC, Guillou F, Gaubicher S, Le Merrer M, Polak M. 2009. Pamidronate treatment of children with moderate-to-severe osteogenesis imperfecta: a note of caution. Horm Res 71:38–44 [DOI] [PubMed] [Google Scholar]
- 74. Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. 2004. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res 19:1779–1786 [DOI] [PubMed] [Google Scholar]
- 75. Sellmeyer DE. 2010. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 304:1480–1484 [DOI] [PubMed] [Google Scholar]
- 76. Al Muderis M, Azzopardi T, Cundy P. 2007. Zebra lines of pamidronate therapy in children. J Bone Joint Surg Am 89:1511–1516 [DOI] [PubMed] [Google Scholar]
- 77. van Persijn van Meerten EL, Kroon HM, Papapoulos SE. 1992. Epi- and metaphyseal changes in children caused by administration of bisphosphonates. Radiology 184:249–254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.