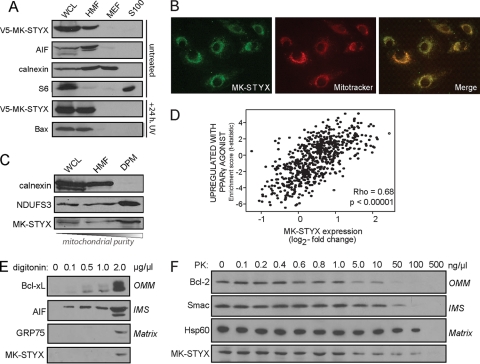
Fig. 8.
MK-STYX localizes to the mitochondria. (A) V5–MK-STYX was transfected into HeLa cells, and cellular compartments were isolated using differential centrifugation: WCL, whole-cell lysate; HMF, heavy membrane fraction/mitochondrion enriched; MEF, microsome-enriched fraction; and S100, cytosol. The fractions were probed with specific markers of each cellular compartment: AIF (mitochondria), calnexin (microsomes), and S6 (cytosol). MK-STYX localization was examined basally (top four gels) and in the context of UV irradiation (bottom two gels). Apoptotic stress is indicated by the translocation of Bax from the cytosol (S100) to the mitochondria (HMF) (bottom gel). (B) The mitochondrial localization of MK-STYX was confirmed using an endogenous antibody raised against MK-STYX (green), which was overlaid with Mitotracker (red; overlay, yellow). (C) Mitochondria were purified from the heavy membrane fraction using a density gradient. DPM, density-purified mitochondria. Pure fractions were probed with calnexin (microsomes) and NDUFS3 (mitochondria) and an antibody against endogenous MK-STYX. (D) Plot of gene set enrichment statistics and STYXL1 expression across tumor gene expression data. MK-STYX has a significant correlation (P < 0.00005) with the “upregulated with PPARγ agonist” gene expression set (R = 0.68), which is highly enriched in mitochondrial genes. (E and F) MK-STYX sublocalization was assayed using increasing digitonin concentrations to differentially release mitochondrial proteins from different compartments (E) and using a dose response of proteinase K, which can access different mitochondrial compartments as concentrations increase (F).