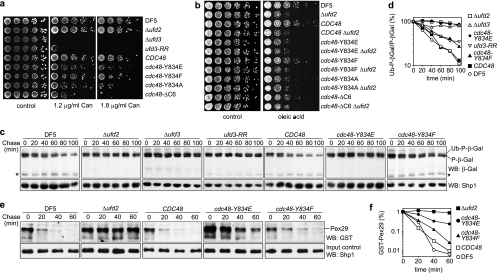
Fig. 6.
cdc48 mutants deficient in Ufd2 and Ufd3 binding phenocopy Δufd2 and Δufd3 mutants. (a and b) Sensitivity to canavanine and oleic acid. Cultures of the indicated wild-type and mutant strains were serially diluted and spotted onto agar plates containing the indicated canavanine (Can) concentrations (a) or 0.2% oleic acid (b) or the respective control plates. Plates were incubated at 30°C (a) or 33°C (b) for 4 days. ufd3-RR, ufd3 mutant strain expressing Ufd3-R541A,R669A impaired in Cdc48 binding. (c) Degradation of a UFD substrate. Protein expression in logarithmically growing cultures of the indicated wild-type and mutant strains expressing ubiquitin-proline-β-galactosidase (Ub-P-β-Gal) under the control of a galactose-induced promoter was stopped by transfer to glucose-containing medium and addition of cycloheximide. The degradation of Ub-P-β-Gal over time was analyzed by Western blot analysis. Note the existence of a very long-lived P-β-Gal and of a metastable β-Gal degradation product (asterisk). A Western blot against Shp1 served as a loading control. (d) Quantification of the results in panel c. The signal of Ub-P-β-Gal was normalized to the signal of the stable P-β-Gal species and plotted against time. (e) Degradation of a bona fide ERAD substrate. Degradation of GST-His6-Pex29 expressed under the control of a galactose-induced promoter was analyzed as described above. At the indicated time points, GST-His6-Pex29 was pulled down with glutathione-Sepharose beads and detected in Western blots against GST. A Western blot against Shp1 served as a control for equal input of cell lysates into the pulldown. Note that GST-His6-Pex29 could be detected only after enrichment by pulldown and not directly in the lysates. (f) Quantification of results in panel e.