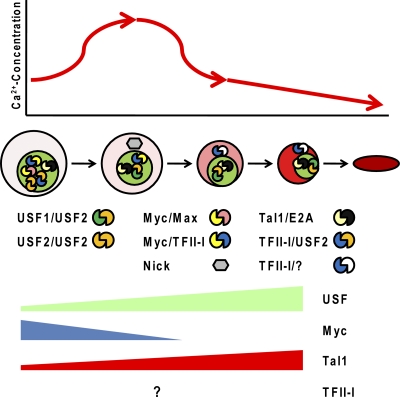
Fig. 5.
Model outlining a correlation of erythroid cell differentiation, calcium concentration, and HLH transcription factor activity. During the differentiation of erythroid cells, there is a transient increase in the intracellular calcium concentration. This activates m-calpain, causing proteolytic cleavage of USF and Myc. The Myc cleavage product Nick, localized in the cytoplasm, may regulate processes involved in the differentiation of erythroid cells. At later erythroid cell differentiation stages, the expression of Myc is repressed. The decrease in the calcium concentration, which may or may not be regulated by the relocation of TFII-I to the cytoplasm, inhibits calpain activity, allowing increased expression of full-length USF. Tal1 expression increases during the differentiation of erythroid cells, but proteolytic degradation in erythroid progenitor cells likely facilitates the conversion of Tal1 from a repressor to an activator. If and how the expression of TFII-I changes during erythroid cell maturation is unknown. It is postulated that during the early stages of erythropoiesis, TFII-I, perhaps in conjunction with USF or Myc, functions as a repressor of erythroid cell-specific genes.