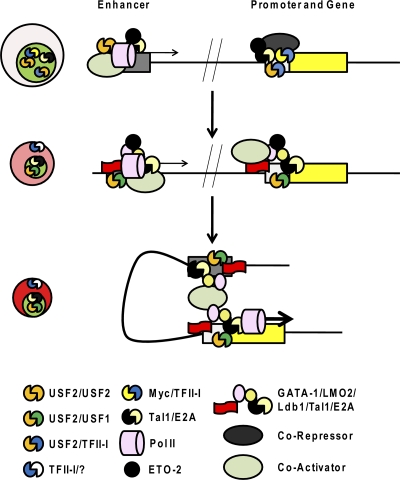
Fig. 6.
Hypothetical model of the regulation of an erythroid cell-specific gene by HLH proteins during the differentiation of erythroid cells. It is proposed that USF2 homodimers and Tal-1/E-protein heterodimers occupy enhancer elements of erythroid cell-specific genes in erythroid progenitor cells. At this stage, Pol II is recruited at low levels and enhancer transcription may assist in maintaining an open configuration (65, 75). The Tal1/E-protein/ETO complex and TFII-I/Myc heterodimers bind to the promoter regions and, through corepressors, keep the promoter in an inactive configuration. During the differentiation of erythroid cells, both enhancer and promoter regions are sequentially activated, which is facilitated by the following processes: (i) relocation of TFII-I to the cytoplasm, (ii) repression of Myc expression, (iii) an increase in the activity of USF, and (iv) conversion of Tal1 from a repressor to an activator. This leads to increased binding of activating HLH proteins, including USF heterodimers and Tal1/GATA-1/LMO2/Lbd1, as well as coactivators, to the enhancer and the promoter. Ldb1 and other activities then mediate proximity between the enhancer and promoter, and activities are transferred from the enhancer to the promoter to position transcription complexes, to enhance transcription elongation, and/or to facilitate reinitiation of transcription. This is a simplified scenario that, in reality, involves many other erythroid cell-specific and ubiquitous activities.