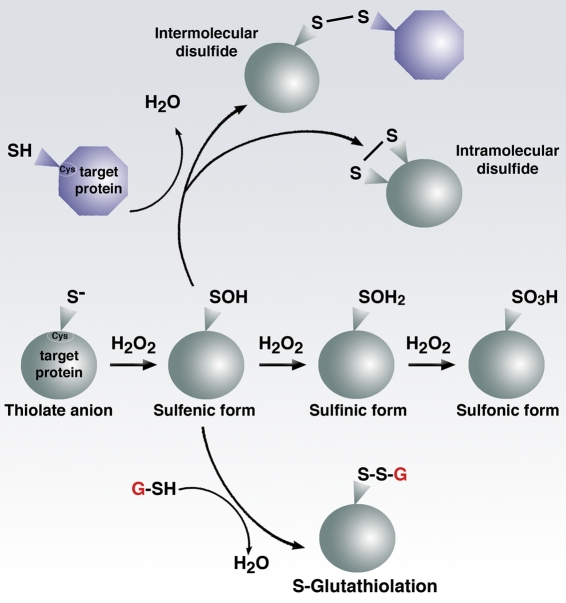
Figure 2.
Cysteine biochemistry allows for redox-dependent signaling. Specific reactive cysteine (Cys) residues within target proteins can be covalently modified by oxidative stress. Much like phosphorylation on serine or threonine residues, alteration of the thiol group can in turn modify enzymatic activity. Although the sulfenic form (SOH) is readily reversible, higher states of oxidation generally, but not always, lead to irreversible modification.