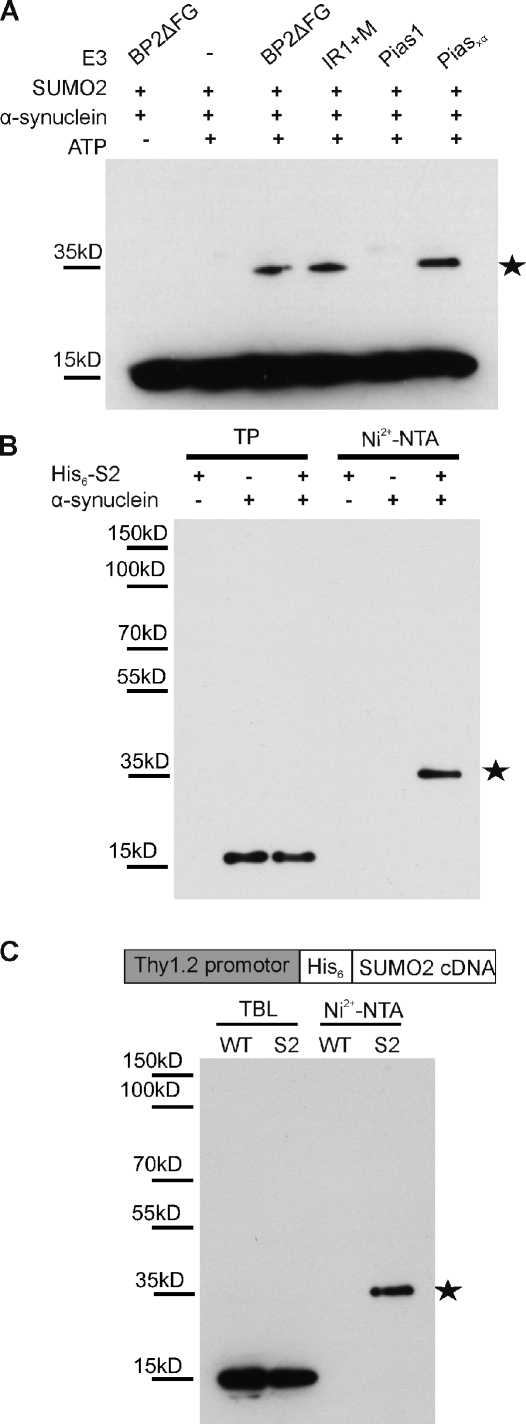
Figure 1.
α-Synuclein sumoylation in vitro in cells and in mouse brain. (A) α-Synuclein is SUMO2 modified in vitro. 500 ng α-synuclein, 500 ng SUMO2, 150 ng Aos1/Uba2, 200 ng Ubc9, and 5–10 ng E3 ligase fragments were incubated for 30 min at 30°C with and without ATP in a volume of 20 µl. Reactions were stopped with SDS sample buffer before analysis by SDS-PAGE and immunoblotting with mouse monoclonal anti–α-synuclein antibody (Syn211; Invitrogen). (B) α-Synuclein modification by SUMO2 in HEK293T cells. Plasmids encoding His6-SUMO2 (His6-S2) or/and α-synuclein were transfected in HEK293T cells. SUMO substrates were purified by Ni2+ affinity chromatography (Ni2+-NTA) under denaturing conditions, and a sumoylated α-synuclein band at ∼35 kD (stars in A and B) was detected with an α-synuclein antibody (Syn211). 1% of total input (TP) and 25% of elution fractions were loaded. (C, top) A schematic representation of the Thy1.2/His6-SUMO transgene. (bottom) α-Synuclein sumoylation in mouse brain tissue. Total His6-SUMO2–conjugated proteins were isolated using Ni2+-NTA affinity chromatography. TBLs and eluates were probed with anti–α-synuclein antibody recognizing mouse α-synuclein (clone 42). α-Synuclein modified by a single SUMO2 molecule, indicated with a star, could be detected in the eluate obtained from His6-SUMO2 transgenics.