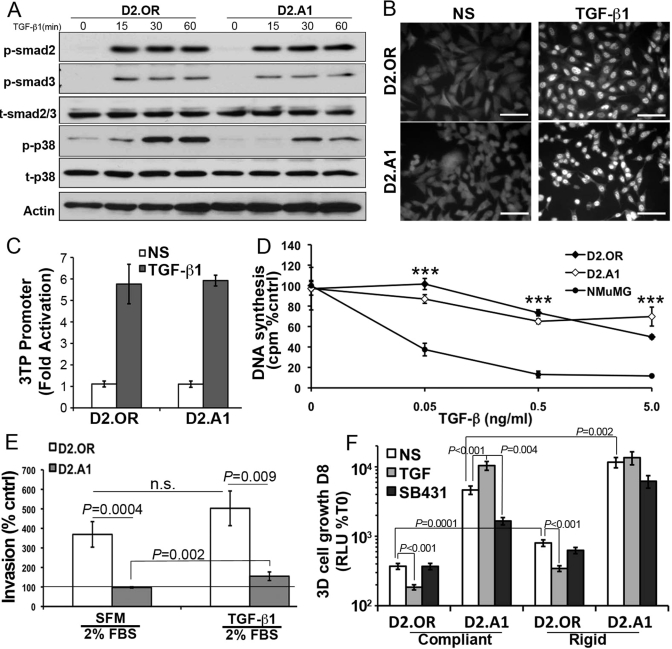
FIGURE 5:
3D culture is required to manifest the TGF-β paradox. (A) Quiescent D2.OR or D2.A1 cells were stimulated with TGF-β1 (5 ng/ml) for the indicated times and analyzed for the presence of phospho-Smad2 (p-smad2), phospho-Smad3 (p-smad3), and phospho-p38 mitogen-activated protein kinase (p-p38). Total Smad2/3 (t-smad2/3), total p38 (t-p38), and actin served as loading controls. Shown are representative immunoblots of three independent experiments. (B) Quiescent D2.OR and D2.A1 cells were stimulated with TGF-β1 as in (B) for 30 min before being processed for Smad2/3 immunofluorescence (400×). Data are representative of 10 random fields of view over two independent experiments. (C) D2.OR and D2.A1 cells were transfected with CMV-control and TGF-βdriven 3TP reporter constructs, and subsequently stimulated with TGF-β1 (5 ng/ml) for 18 h. Data are the mean (±SE) of three independent experiments completed in triplicate. (D) D2.OR, D2.A1, and NMuMG cells were incubated for 48 h in the absence or presence of increasing concentrations of TGF-β1 as indicated. Incorporation of [3H]thymidine was determined as a measure of DNA synthesis. Data are normalized to untreated controls and are the mean (±SE) of three independent experiments completed in triplicate. ***, p < 0.001. (E) D2.OR or D2.A1 cells were inoculated into the top well of an invasion assay in the absence or presence of TGF-β1 (5 ng/ml). Data are normalized to serum-free medium (line) and are the mean (±SE) of two independent experiments completed in triplicate. n.s., not significant. (F) 3D outgrowth of D2.OR and D2.A1 cells under compliant and rigid conditions was quantified by bioluminescence. Where indicated, the cells were grown in the presence of TGF-β1 (5 ng/ml) or the TβR-I inhibitor, SB431542 (SB431, 10 μM). Data are the mean (±SE) of at least two independent experiments completed in triplicate.