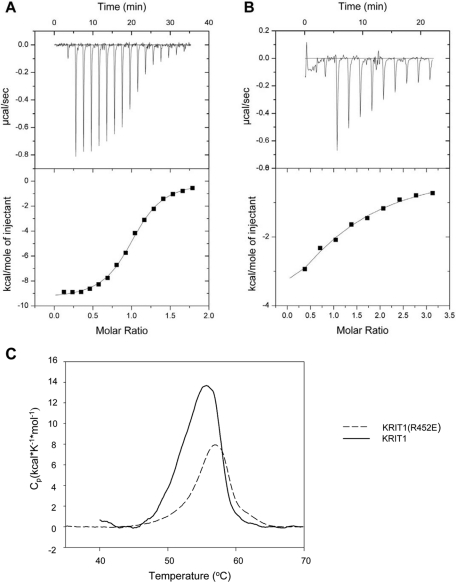
FIGURE 3:
Calorimetric characterization of KRIT1 FERM-domain binding to Rap1B bound to GMP-PNP, a GTP analogue. (A) Calorimetric titration of 400 μM Rap1B, out of the syringe, into 45 μM wild-type KRIT1 FERM domain in the sample cell. (B) Titration of 1.2 mM Rap1B into 45 μM KRIT1(R452E) FERM domain mutant protein. (C) KRIT1(R452E) FERM mutant does not disrupt protein folding. Differential scanning calorimetry results of wild-type and R452E FERM proteins both exhibit similar narrowly defined melting points, indicating that they are well folded.