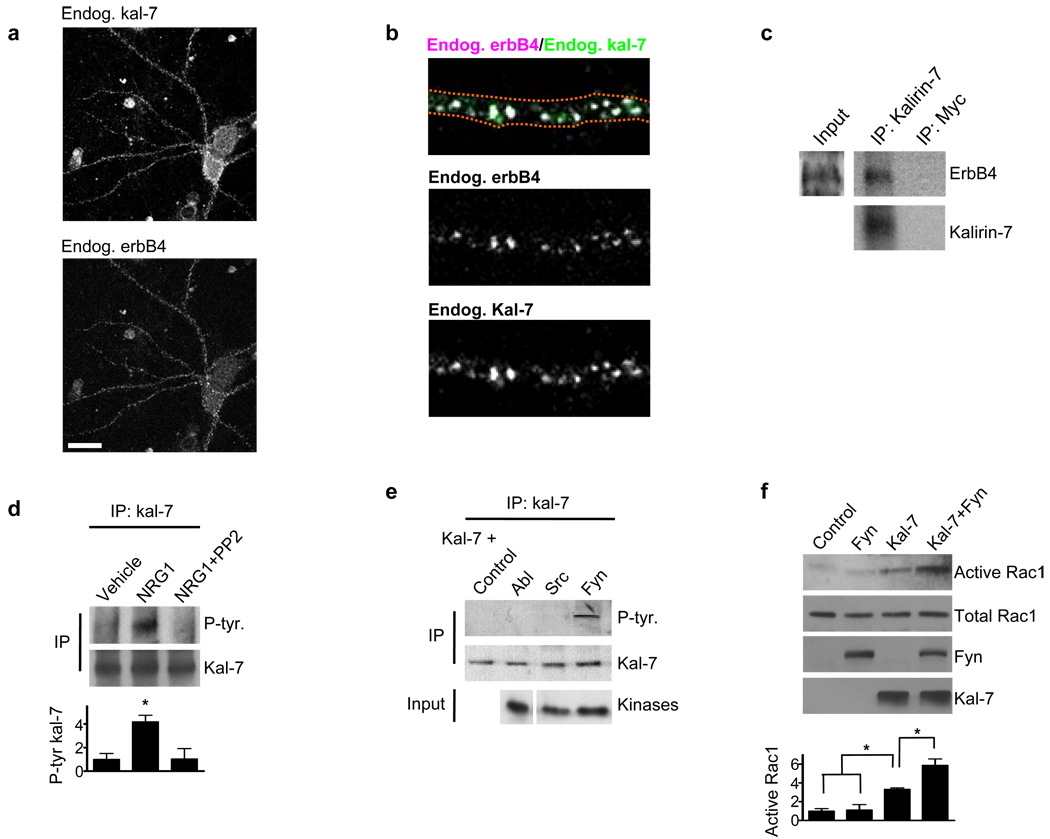
Figure 2. Kalirin-7 and erbB4 interact and fyn phospho-activates kalirin-7.
(a) DIV28 cortical cultures were immunostained for endogenous erbB4 and endogenous kalirin-7. Immunostaining shows a high degree of colocalization between kalirin-7 and erbB4 in interneurons. Scale bar=35uM.
(b) 71±4.6% of erbb4 puncta in mature interneuronal dendrites colocalized with kalirin-7.
(c) Kalirin-7 was immunoprecipitated from mature cortical cultures and the immunoprecipitate probed for erbB4. Myc was used as an immunoprecipitation control. Kalirin-7 interacted with erbB4.
(d) Kalirin-7 was immunoprecipitated from mature cortical cultures and the immunoprecipitate probed with a phospho-tyrosine antibody. Whereas NRG1β (5nM, 3-days) treatment increased kalirin-7 tyrosine phosphorylation, co-treatment with PP2 (10uM) blocked this effect. Graph displays change relative to the vehicle condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05
(e) hEK293 cells overexpressing various kinases in combination with kalirin-7. Kalirin-7 was immunoprecipitated and the immunoprecipitate probed with a phospho-tyrosine antibody. Of the tested tyrosine kinases, only fyn phosphorylated kalirin-7.
(f) hEK293 cells overexpressing fyn and kalirin-7 individually or together, and active Rac1 levels assessed. Fyn in combination with kalirin-7 increased active Rac1 levels beyond that of kalirin-7 or fyn alone. Graph displays change relative to the control condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05