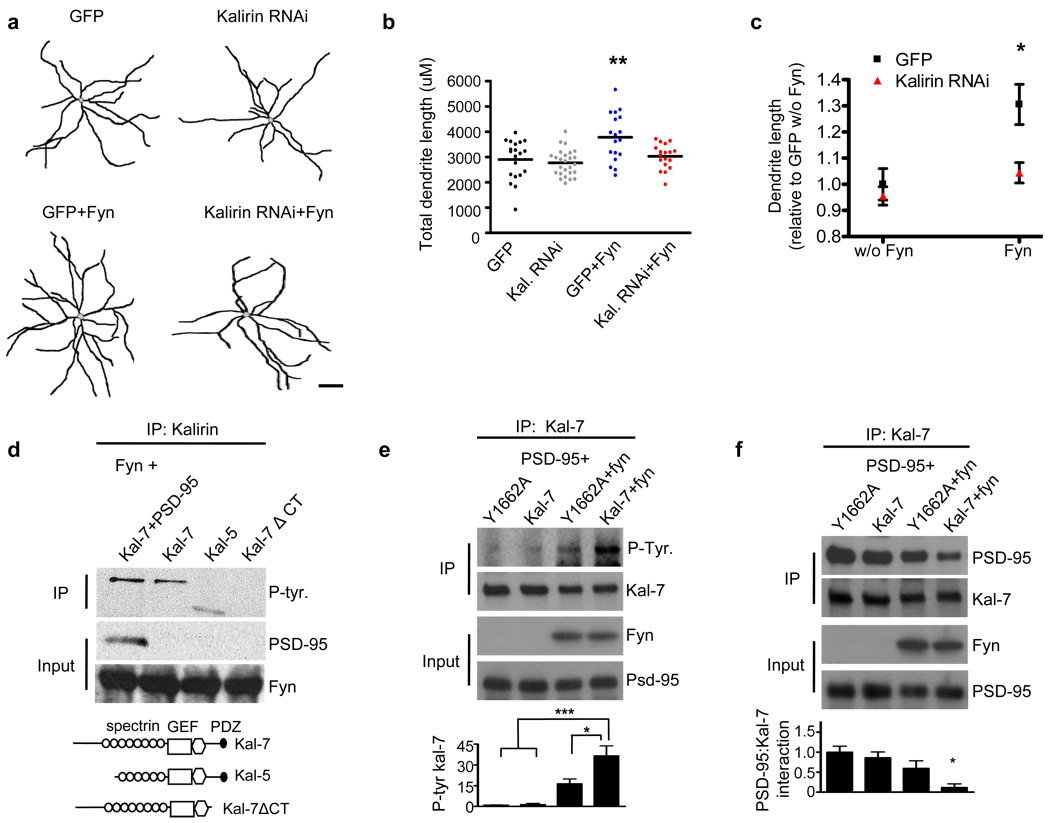
Figure 3. Fyn increases interneuronal dendritic growth through kalirin, and fyn phosphorylates kalirin-7 through its C-terminus.
(a) Representative traces of DIV28 cultured cortical multipolar interneuron dendritic trees. Axons were omitted for clarity. Neurons were transfected with either GFP, GFP-tagged kalirin RNAi, fyn, or GFP-tagged kalirin RNAi in combination with fyn. Scale bar=100um.
(b) Scatter plots showing that fyn overexpression increases interneuronal dendritic length. The knockdown of kalirin blocked the effects of fyn on interneuron dendritic length. Knockdown of kalirin by itself did not affect dendritic length. Each point represents the total dendritic length of a single cell. Significance determined using a one-way ANOVA with Tukey post-hoc. Black lines are the mean, n=18–26 cells per condition; **p<0.01
(c) Significant interaction of fyn expression by kalirin expression with regard to total interneuron dendritic length. Significance determined using 2×2 ANOVA and the interaction term determined. Data are the mean±SEM; *p<0.05
(d) Domain mapping in hEK293 cells overexpressing various kalirin constructs in combination with fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed with a phospho-tyrosine antibody. Fyn phosphorylated kalirin-7 in the presence and absence of PSD-95, and fyn phosphorylated kalirin-5. Fyn failed to phosphorylate the kalirin-7 ΔCT construct. Schematics of kalirin constructs used are shown below.
(e) hEK293 cells overexpressing Y1662A or WT kalirin-7 together with PSD-95, with or without fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed for phosphotyrosine. Fyn strongly phosphorylated kalirin-7. Mutation of the Y1662 kalirin-7 residue to alanine (Y1662A) significantly reduced fyn-mediated phosphorylation. Graph displays change relative to the Y1662A condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=4 independent experiments; *p<0.05, ***p<0.001
(f) hEK293 cells overexpressing Y1662A or WT kalirin-7 together with PSD-95, with or without fyn. Kalirin was immunoprecipitated and the immunoprecipitate probed for PSD-95. WT kalirin-7 and the Y1662A mutant showed a similar magnitude of interaction with PSD-95 in the absence of fyn. However, fyn reduced the association of WT kalirin-7, but not Y1662A kalirin-7, with PSD-95. Graph displays change relative to the Y1662A condition and significance determined using a one-way ANOVA with Tukey post-hoc. Data are the mean±SEM, n=3 independent experiments; *p<0.05