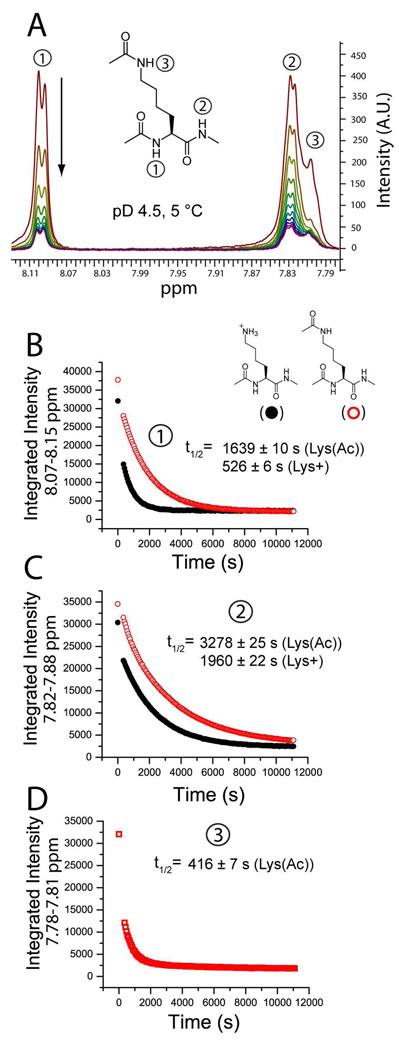
Figure 6. Neutralizing the ε-NH3+ group of Ac-Lys(ε-NH3+)-NHMe reduces the rate of H/D exchange at the backbone amide of the amino acid.
The rate of backbone amide exchange for model lysine compounds Ac-Lys(ε-NH3+)-NHMe and Ac-Lys(ε-NHCOCH3)-NHMe were measured with 1H NMR spectroscopy at pD 4.5, 5°C. A) The exchange of the amide hydrogen of the α-nitrogen (e.g., the ‘left-handed’ amide) was monitored in both compounds by the disappearance of the signal at ~ 8.10 ppm and the ‘right-handed’ amide by the signal at ~ 7.83 ppm. The ε-amide hydrogen (ε-NHCOCH3) of Ac-Lys(ε-NHCOCH3)-NHMe appears at ~ 7.80 ppm. B–D). The amide H/D exchange of both compounds expressed as a function of the integrated signal and time. Solid black circles represent Ac-Lys(ε-NH3+)-NHMe and open red circles represent the neutral compound Ac-Lys(ε-NHCOCH3)-NHMe. The function y = yo + Ae(-x/k) was fit to each plot. B) For the left handed amide (denoted “1”), the half-life of exchange (t1/2) = 526 ± 6 s for Ac-Lys(ε-NH3+)-NHMe (R2 = 0.9897) and 1639 ± 10 s for Ac-Lys(ε-NHCOCH3)-NHMe (R2 =0.9985). The ratio of half lives = 3.1. C) The neutralization of ε-NH3+ decreased the rate of exchange of the right handed amide (denoted “2”) by a lesser degree than the left handed amide. For Ac-Lys(ε-NH3+)-NHMe t1/2 = 1960 ± 22 s (R2 = 0.9958) and for Ac-Lys(ε-NHCOCH3)-NHMe t1/2 = 3278 ± 25 s (R2 = 0.9989); the ratio of half lives = 1.7. D) The ε-amide of Ac-Lys(ε-NHCOCH3)-NHMe (denoted “3”) exchanges faster than any of the backbone amide hydrogen: t1/2 = 416 ± 7 s.