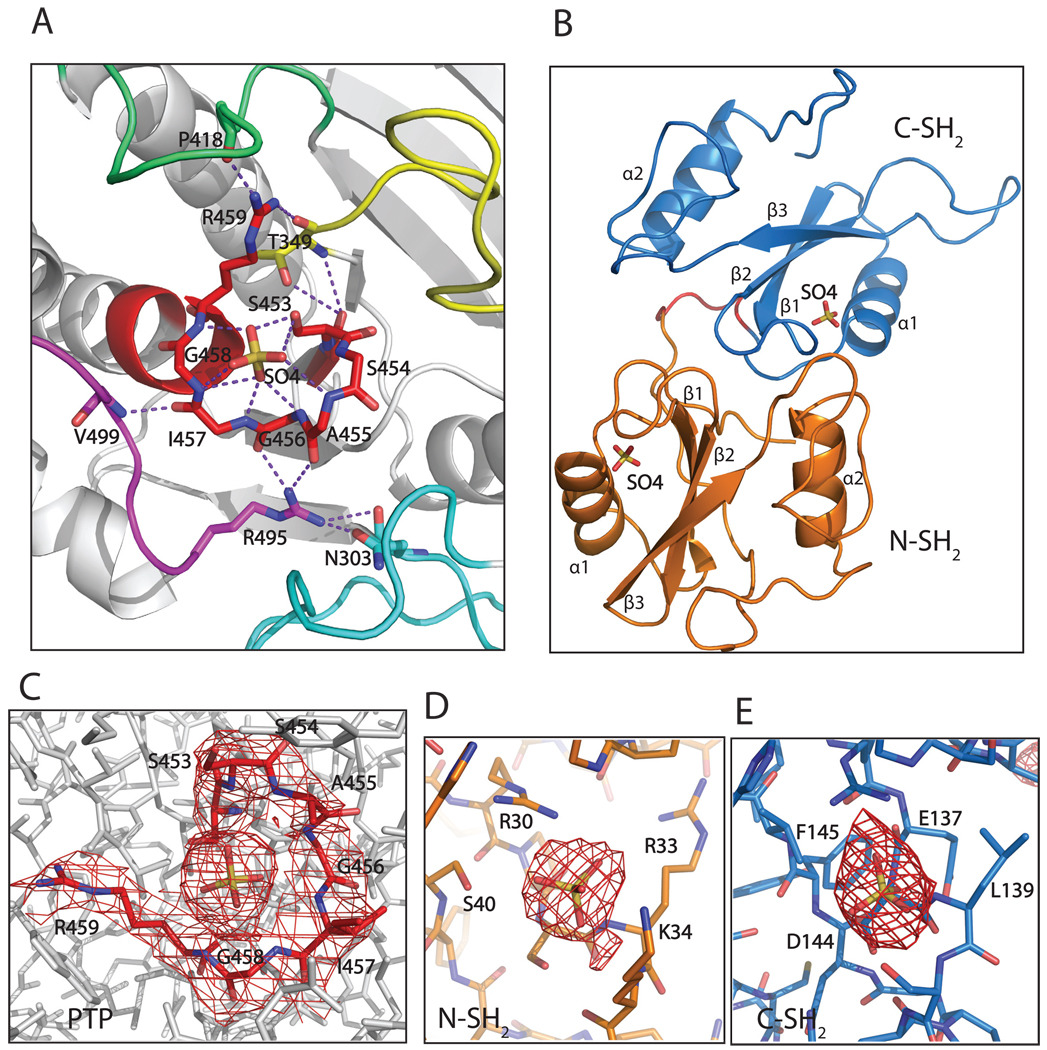
Figure 2.
The ligand-binding site of each domain has a bound sulfate ion. A. The active site (in red) has a sulfate ion, which is stabilized by surface loops: the loop between strands β3 and β4 (yellow); the WPD loop between helix α3 and strand β7 (lime green); the loop between helices α5 and α6 (magenta), and indirectly by the loop between helix α1 and strand β1 (cyan). Residues N303, T349, P418, R495 and V499 from these loops contribute to the stabilization of the active site. B. A sulfate ion was observed in the binding site of each SH2 domain as well. C–E. Electron density maps around the active site and the sulfates in the binding pocket of PTP, and around the sulfates in the binding sites of N-SH2 and C-SH2 domains. The 2Fo-Fc maps are contoured at 1σ level.