Table 1.
Optimization of Reaction Conditions for Pd-Catalyzed C(sp3)–H Carbonylationa
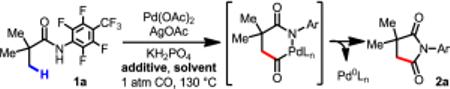
| entry | additive | solvent | yield (%)b |
|---|---|---|---|
| 1 | none | DMF | <1 |
| 2 | none | toluene | 7 |
| 3 | none | C6F6 | 8 |
| 4 | none | n-hexane | 30 |
| 5 | BQ | n-hexane | 13 |
| 6 | Cu(OAc)2 | n-hexane | 4 |
| 7 | DMF | n-hexane | 54 |
| 8 | PivOH | n-hexane | 50 |
| 9 | TEMPOc | n-hexane | 80 |
| 10 | TEMPO | n-hexane | 95 |
Reaction conditions: 1a (0.1 mmol), Pd(OAc)2 (10 mol %), AgOAc (2.0 equiv), KH2PO4 (2.0 equiv), additive (2.0 equiv), solvent (1 mL), CO (1 atm), 130 °C, 18 h.
The yield was determined by 1H NMR analysis of the crude product using 1,1,2,2-tetrachloroethane as an internal standard.
TEMPO (0.2 equiv).
