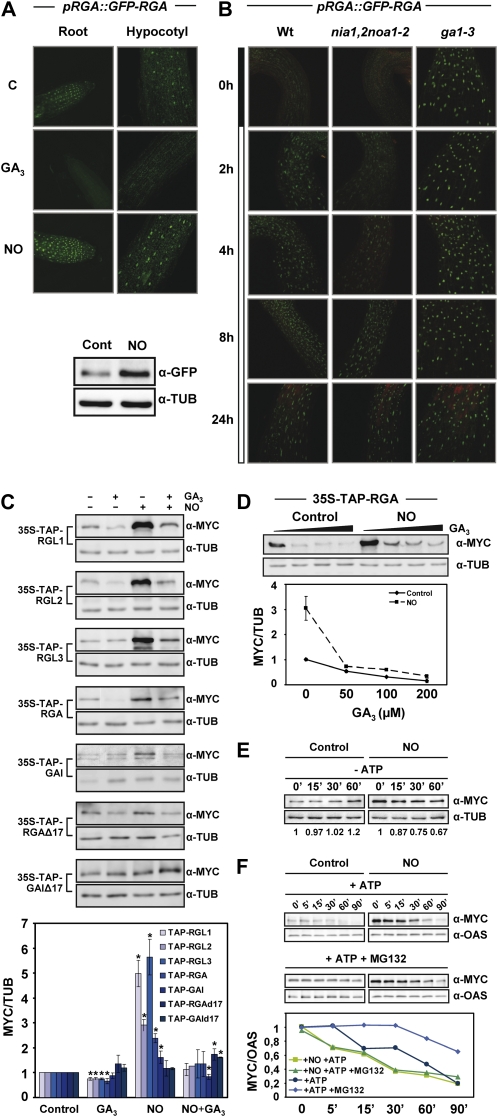
Figure 4.
Effect of NO on DELLA protein accumulation. A, GFP-RGA in pRGA::GFP-RGA roots and hypocotyls, either untreated (control [C]) or treated for 2 h with 50 μm GA3 or 250 μm SNP as a source of NO, visualized by confocal microscopy. GFP-RGA levels and the loading control tubulin (TUB) were analyzed by western blot. B, GFP-RGA protein in hypocotyls of pRGA::GFP-RGA in wild-type, nia1,2noa1-2, and ga1-3 backgrounds at different times after the shift from darkness to red light, as indicated in the bar at left. C, TAP-tagged versions of every DELLA protein were used to analyze the levels of each protein in seedlings treated (+) or not (−) with 50 μm GA3 and/or 250 μm SNP (NO) for 2.5 h. TAP-DELLAs were detected with anti-MYC antibodies, and the levels of tubulin are shown as a loading control. The values normalized with tubulin and relative to control untreated samples were quantified, and the values shown in the bottom panel correspond to means of three independent experiments ± sd. Asterisks represent statistically significant differential values with at least P < 0.05 when comparing treated versus untreated controls in each genotype. D, GA-induced degradation of RGA in untreated (Control) and NO-treated (NO; as described in C) 35S-TAP-RGA seedlings exposed to increasing GA3 concentrations (0, 50, 100, and 200 μm). Values normalized to tubulin were quantified and are shown in the bottom panel as means of three independent experiments ± se. E and F, Cell-free degradation assay of RGA in the absence (E) or presence (F) of ATP and the proteasome inhibitor MG132. Protein samples were incubated at room temperature for the indicated times and treatment conditions and detected with anti-MYC antibodies. Tubulin or O-acetyl-Ser(thiol)lyase 1 (OAS) was detected as a loading control (D–F). ATP and MG132 were used at 10 mm and 100 μm, respectively. The protein levels detected on western blots in E and F were quantified and normalized to TUB or OAS content.