Abstract
Rationale
The coordination of vascular smooth muscle cell (VSMC) constriction plays an important role in vascular function such as regulation of blood pressure. However, the mechanism responsible for VSMC communication is not clear in the resistance vasculature. Pannexins (Panx) are purine releasing channels permeable to the vasoconstrictor ATP and thus may play a role in the coordination of VSMC constriction.
Objective
We investigated the role of pannexins in phenylephrine (PE) and KCl mediated constriction of resistance arteries.
Methods and Results
Western blot, immunohistochemistry and immunogold labeling coupled to scanning and transmission electron microscopy revealed the presence of Panx1 but not Panx2 or Panx3 in thoracodorsal resistance arteries (TDA). Functionally, the contractile response of pressurized TDA to PE was significantly decreased by multiple Panx inhibitors (mefloquine, probenecid and 10Panx1), ectonucleotidase (apyrase) and purinergic receptor inhibitors (suramin and reactive-blue-2). Electroporation of TDA with either Panx1-GFP or Panx1 siRNA showed enhanced and decreased constriction respectively in response to PE. Lastly, the Panx inhibitors did not alter constriction in response to KCl. This result is consistent with co-immunoprecipitation experiments from TDA, which suggested an association between Panx1 and α1D-adrenoreceptor.
Conclusions
Our data demonstrate for the first time a key role for Panx1 in resistance arteries, by contributing to the coordination of VSMC constriction and possibly regulation of blood pressure.
Keywords: pannexins, phenylephrine, adrenergic receptor, smooth muscle cells, vasoconstriction
Introduction
In resistance arteries, the coordination of vascular smooth muscle cell (VSMC) constriction helps to regulate blood flow and peripheral resistance, and thus overall blood pressure1. Although it has been hypothesized that gap junctions link VSMC in resistance arteries to provide this coordination, the gap junctional coupling of VSMC is controversial3, and the presence of gap junctions and the gap junction proteins (connexins) are difficult to observe4. Thus, VSMC may use other mechanisms to communicate and coordinate their responses.
Pannexins (Panx) are tetra-spanning membrane proteins that mediate paracrine intercellular communication via release of purines such as ATP or UTP 5, 6. The physiological function of Panx remains poorly documented in the vasculature where there is currently no indication of Panx expression or function. We thus hypothesized that pannexins may coordinate VSMC constriction through the release of purines and activation of purinergic receptors.
Materials and Methods
See supplemental material for expanded methods.
Mice
Male mice (12–16 weeks) were used according to the University of Virginia Animal Care and Use Committee guidelines.
Electron microscopy
Thoracodorsal resistance arteries (TDA) were immunolabeled using Panx1 antibody and 10 nm gold beads and imaged using a JEOL 6400 scanning electron microscope.
Vessel transfection
The VSMC of TDA were transfected either with a plasmid containing Panx1-GFP or Panx1 siRNA and cultured for 16 to 18 hours.
Co-immunoprecipitation
Panx1 or α1D-adrenoreceptor antibody were conjugated to Dynabeads, incubated with TDA lysates and run on a SDS-PAGE gel.
Statistics
1-way or 2-way ANOVA followed by Bonferroni’s post-test were used for comparisons between treatments. A P value of <0.05 was significant.
Results
Panx1 is expressed in VSMC of TDA
Using transmission electron microscopy (TEM), we could not observe gap junctions between VSMC in TDA but could clearly identify tight junctions between endothelial cells (EC; Fig 1A). The absence of membrane apposition was confirmed in other resistant arteries while gap junctions could be observed between VSMC in mouse aorta (Suppl Fig I). Therefore, we tested for the presence of the three Panx isoforms in TDA. Using western blot (Fig 1B) and immunolabeling, we could only identify Panx1 both in EC and VSMC, and between VSMC (Fig 1C-D-E). In mouse aorta, we could not detect any Panx isoform in the VSMC (Suppl Fig I). Immuno-SEM on TDA using an extracellular loop Panx1 antibody revealed the presence of the protein on the VSMC plasma membrane (Fig 1F).
Figure 1. Panx1 is expressed in the membrane of VSMC in TDA.
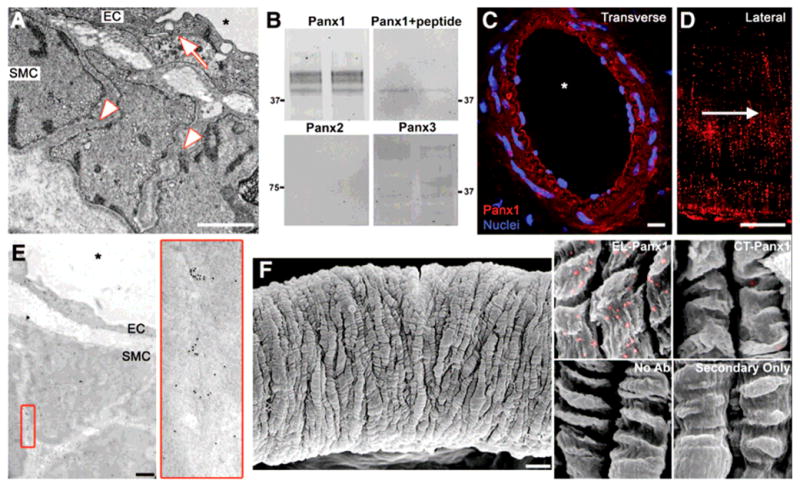
(A) Representative TEM image of TDA demonstrates close apposition (arrow) between EC, whereas VSMC are separated by a large intercellular space (arrowheads). (B) Duplicate western blots of TDA lysates for the three Panx isoforms (Panx1, Panx2 and Panx3) and Panx1 antibody incubated with cognate peptide. (C) immunofluorescence of a transverse section of TDA labeled for Panx1 (red), nuclei are stained with DAPI (blue). (D) Immunofluorescence of a lateral view of VSMC of a TDA labeled for Panx1 (red), arrow indicates direction of blood flow. (E) Immuno-TEM labeling of TDA for Panx1; enlargement of red box is shown on right. (F) Left: representative SEM of a TDA. Right: enlarged images of VSMC from immuno-SEM labeled for Panx1 (gold beads were pseudocolored in pink). Samples were labeled with antibodies against the extracellular loop of Panx1 (EL-Panx1) or the C-terminal of Panx1 (CT-Panx1); VSMC without labeling or primary antibodies are also shown. Scale bar is 1μm in (A) and (E), 5μm in (D), 10μm in (C) and (F). Asterisks indicate vessel lumen.
Phenylephrine-induced constriction is mediated by Panx
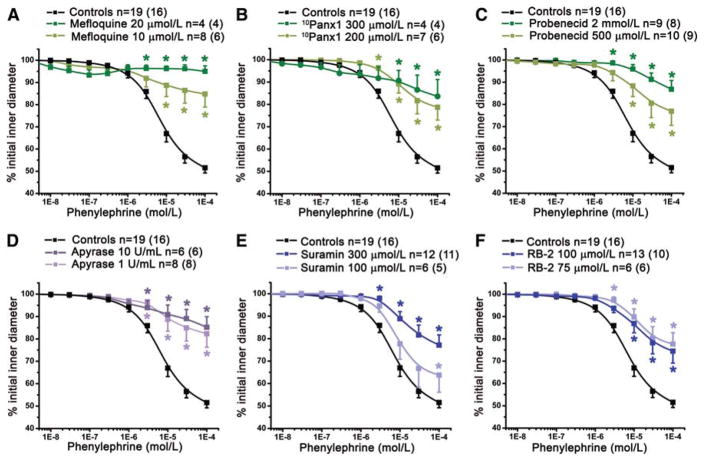
The data above clearly demonstrate that Panx1 is expressed in VSMC, however its functional role in the resistance arteries is unknown. We therefore performed dose response curves using the α1-adrenoreceptor agonist phenylephrine (PE) in the presence of three Panx inhibitors: mefloquine7 (Fig 2A), 10Panx1 peptide8, 9 (Fig 2B), and probenecid6, 9 (Fig 2C). Panx inhibitors all significantly reduced PE-induced vasoconstriction (Fig 2A-C, Suppl Fig II-III) but did not affect ATP-induced vasoconstriction (Suppl Fig IV).
Figure 2. Panx is involved in PE constriction of TDA.
The effect of cumulative concentrations of PE on internal diameter of pressurized TDA was significantly decreased after treatment with Panx inhibitors: (A) mefloquine (10 μmol/L), (B) 10Panx1 peptide (200 μmol/L) and (C) probenecid (2 mmol/L and 500μmol/L). (D), effect of apyrase (1 U/mL and 10 U/mL). We also inhibited purinergic receptors with suramin (100 μmol/L and 300 μmol/L), (E) and P2Y receptors with RB-2 (75 μmol/L); (F). n indicates the number of vessels and the value in parentheses is the number of mice. *P<0.05.
The Panx channels release purines5, 6, which induce constriction when applied to TDA (Suppl Fig IV). We thus tested the effect of apyrase, an ectonucleotidase that degrades purines. Both 1U/mL and 10U/mL significantly reduced TDA constriction in response to PE (Fig 2D, Suppl Fig II-III). Purines such as ATP and UTP are known to bind purinergic receptors present on VSMC. Therefore we treated the vessel with suramin, a purinergic receptor inhibitor (Fig 2E, Suppl Fig II-III), and Reactive Blue-2 (RB-2; Fig 2F, Suppl Fig II-III), which inhibits the P2Y subfamily of purinergic receptors. Both antagonists significantly inhibited PE-induced constriction. To test whether PE-induced constriction was due to Panx1 localized on EC, we perfused probenecid, apyrase and RB-2 in the lumen of TDA and found no changes of PE responses, a result consistent with experiments on endothelium-denuded TDA where probenecid inhibit PE-induced constriction to the same extent as observed on intact TDA (Suppl Fig V). Lastly, cultured VSMC from coronary resistance arteries (express both α1D-adrenoreceptor and Panx1) had significant increases in ATP release after PE stimulation that was inhibited by 10Panx1, whereas cultured aortic VSMC (only express α1D-adrenoreceptor) and NRK cells (do not express α1D-adrenoreceptor or Panx1) did not have any increase in ATP release upon PE stimulation (Suppl Fig VI).
Modulation of Panx1 expression modifies PE-induced constriction of TDA
The TDA were transfected either with Panx1-GFP or with Panx1 siRNA (Suppl Fig VII). When Panx1 was overexpressed, the response of TDA to PE was increased by approximately 30%, whereas underexpression of Panx1 induced a decreased constriction to PE by 45% (Fig 3A, Suppl Fig VIII). Transfection of TDA with control siRNA did not affect PE-induced constriction (Suppl Fig VII). Immunolabeling of transfected TDA revealed that Panx1 expression was only modified in VSMC and not in EC (Fig 3B), which was confirmed using an anti-GFP antibody (Suppl Fig VII). Endothelial and smooth muscle function were not altered by transfection (Suppl Fig IX).
Figure 3. Modulation of Panx1 expression in VSMC modifies PE-induced constriction of TDA.
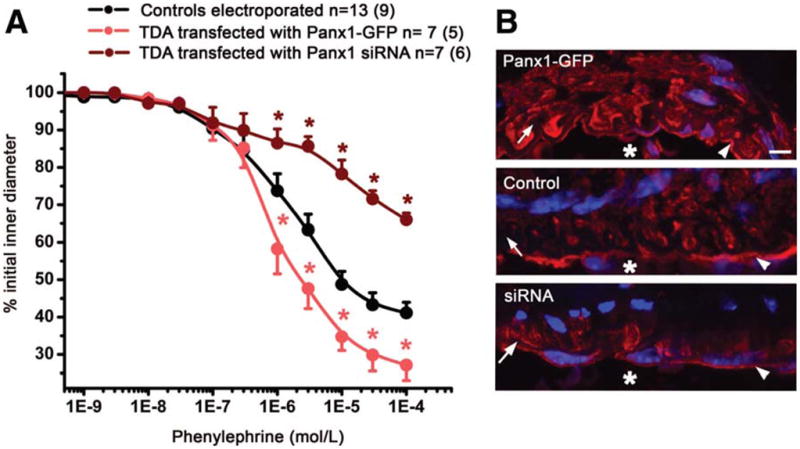
The VSMC of TDA were transfected with either a plasmid containing Panx1-GFP or Panx1 siRNA. (A) The effect of cumulative concentrations of PE was investigated in each condition. (B) Immunofluoresence of TDA transfected with Panx1-GFP (upper panel), untransfected TDA (middle panel) and TDA transfected with Panx1 siRNA (lower panel) labeled for Panx1. Scale bar is 5μm. The asterisks in A indicates P<0.05 and in B, the asterisks indicates vessel lumen. In B, arrows point to IEL, arrowheads point to EC.
Panx1 is associated with α1-adrenoreceptor and is not involved in KCl constriction
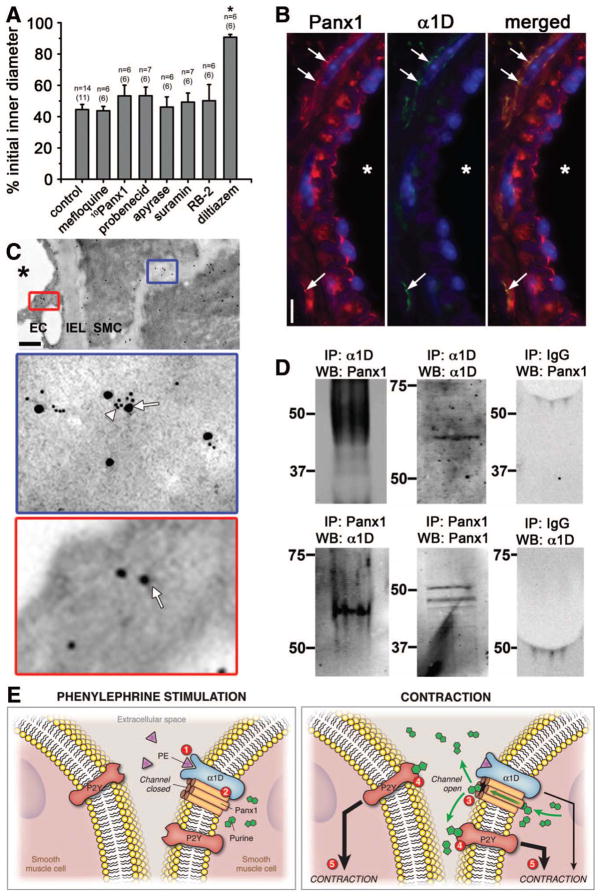
We investigated whether Panx inhibitors could alter a receptor independent stimulus such as KCl. We tested the three different Panx inhibitors as well as the purinergic inhibitors and found that none of them affected TDA constriction to KCl (Fig 4A). We thus hypothesized that a specific interaction may occur between Panx1 and α1D-adrenoreceptor, the primary adrenoreceptor responsible for VSMC constriction (Suppl Fig X). Immunolabeling of TDA using Panx1 and α1D-adrenoreceptor antibodies revealed co-localization of the two proteins (Fig 4B-C). The association between Panx1 and α1D-adrenoreceptor was confirmed by co-immunoprecipitation from TDA lysates (Fig 4D).
Figure 4. Panx1 is associated with α1D -adrenoreceptor and is not involved in KCl constriction.
(A) Previously used inhibitors (mefloquine (10 μmol/L); 10Panx1 (200 μmol/L), probenecid (2 mmol/L) apyrase (10 U/mL), suramin (300 μmol/L) or RB-2 (75 μmol/L)) had no effect on 40 mmol/L KCl-induced constriction. However, the L-type Ca2+ channel inhibitor diltiazem (10 μmol/L) significantly inhibited the KCl-induced constriction. *P<0.05. (B) Immunolabeling of transverse sections of TDA labeled for Panx1 (red) and α1D-adrenoreceptor (green). Nuclei are stained in blue and asterisks indicate the lumen. Arrows point to colocalized labeling for Panx1 and α1D-adrenoreceptor. Scale bar is 10μm. (C) Immunogold labeling of TDA for Panx1 (arrows) and α1D-adrenoreceptors (arrowheads). Scale bar is 1 μm. (D) Interaction between Panx1 with α1D-adrenoreceptors was revealed when lysates from TDA were immunoprecipitated (IP) either with α1D-adrenoreceptor (α1D, upper left panel) or Panx1 (lower left panel) and blotted (WB) with Panx1 or α1D-adrenoreceptor antibodies, respectively. Panx1 and α1D-adrenoreceptor pull down were verified by blotting with Panx1 or α1D-adrenoreceptor antibodies (middle panels) and the absence of IgG was also controlled (right panels). (E) Summary of PE-induced vasoconstriction: application of PE stimulates α1D-adrenoreceptor (1) followed by the activation of Panx1 channel (2) leading to the opening of the Panx1 channel and the release of purines in the extracellular space (3). Purines bind to P2Y receptors (4) reinforcing the α1D-adrenoreceptor constriction (5).
Discussion
In the microcirculation, coordination of VSMC contraction is essential for the regulation of blood flow distribution and peripheral vascular resistance. Gap junctions are likely responsible for VSMC coordination in conduit arteries, however their role in VSMC coordination in resistance arteries remains unclear4. Our TEM results indicate that VSMC of TDA are unlikely to be coupled exclusively via gap junctions since the VSMC are separated by relatively large intercellular spaces. The close apposition of EC plasma membranes, characteristic of gap junctions, indicates that the intercellular space observed between VSMC was not an artifact of fixation. Our demonstration of Panx1 expression could suggest a mechanism allowing for VSMC to coordinate their responses independent of gap junctions.
Pannexin channels release purines in response to different stimuli5. In the vasculature, the concentration of purines is finely regulated by ectonucleotidase at the surface of VSMC and contribute to local regulation of vascular tone10. Our findings suggest that Panx1, purines and purinergic receptors are involved in TDA constriction in response to PE. However, we also found staining for Panx1 in EC, which are known to release ATP11 but our data show that the inhibition of PE responses was not due to Panx1 on EC. Nevertheless, Panx1 expression in EC suggests that Panx1 could be involved in EC functions such as vasodilation or inflammatory cell adhesion.
Our data implies that Panx1 is specifically involved in PE- ,but not KCl-induced constriction. This suggests that Panx1 is not activated solely by the rise of intracellular calcium concentrations or mechanical stretch5. Reports from cell culture have suggested that purine release through Panx1 may occur after activation of Gq/11-coupled α1-adrenoreceptor but not after stimulation of Gi/o-coupled α2-adrenoreceptors or Gs-coupled β-adrenoreceptors12. Thus, Panx1 could also be involved in TDA constriction in response to other Gq/11-coupled receptors agonists such as serotonin, endothelin-1 or angiotensin II. However, our evidence indicates that Panx1 and α1D-adrenoreceptor are closely associated at the protein level, suggesting Panx1 and α1-adrenoreceptor may be part of a signaling microdomain.
Our data provides the first demonstration of Panx expression in the vasculature and its function in the control of vasoconstriction. Our results suggest that the release of purines through Panx1 channels on VSMC is triggered by PE stimulation and participates in control of vascular tone through purinergic receptors (Fig 4D). Thus, Panx1 could contribute to the coordination of VSMC constriction and the regulation of blood pressure via catecholamines released by sympathetic nerves.
Supplementary Material
Novelty and significance.
What is known?
Intercellular communication between smooth muscle cells (SMC) within the vascular wall is essential for the control of vasoreactivity but the mechanism remains unclear.
Pannexin channels participate in intercellular communication through the release of purines such as ATP, a potent vasoconstrictor.
What new information does this article contribute?
Pannexin1 (Panx1) is present in the SMC of resistant arteries and play a role in phenylephrine (PE)-induced vasoconstriction.
Panx1 and the α1D-adrenoreceptor are part of the same protein complex.
When PE binds to the α1D-adrenoreceptor, Panx1 opens to release purines that can act on purinergic receptors present on SMC to enhance PE-induced vasoconstriction.
Intercellular communication between SMC serves to regulate the vasoconstriction of resistance arteries, a fundamental process for the control of blood flow and peripheral resistance. However, the exact mechanisms of SMC communication remain unclear. Here we focused on Panx1, a protein known to participate in the release of ATP which has not been described in the vasculature. Our data indicate that Panx1 releases ATP during contraction of resistance arteries in response to PE, therefore controlling the intensity of vasoconstriction. These data are the first to describe the presence, and a role for, Panx1 in the vascular wall providing a novel mechanism for SMC communication.
Acknowledgments
We thank Doug Bayliss and Brian Duling for critical reading of the manuscript; Allison Armstrong for assistance with the ATP assay; the University of Virginia Histology Core for sectioning; Anita Impagliazzo for the illustration; and Jan Redick and Stacey Guillot at the University of Virginia Advanced Microscopy Core.
Sources of Funding
This work was supported by NIH HL088554 (BEI), AHA SDG (BEI), an AHA post-doctoral fellowship (MB; SRJ), an NRSA post-doctoral fellowship (ACS), R15 HL102742-01 (RLW) and CIHR (DWL).
Non-standard Abbreviations and Acronyms
- EC
Endothelial Cells
- NRK
Normal Rat Kidney
- PE
Phenylephrine
- Panx
Pannexin
- RB-2
Reactive Blue-2
- SEM
Scanning Electron Microscopy
- TDA
Thoracodorsal Artery
- TEM
Transmission Electron Microscopy
- VSMC
Vascular Smooth Muscle Cells
Footnotes
Disclosures
None.
References
- 1.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 2.Fanchaouy M, Serir K, Meister JJ, Beny JL, Bychkov R. Intercellular communication: role of gap junctions in establishing the pattern of ATP-elicited Ca2+ oscillations and Ca2+-dependent currents in freshly isolated aortic smooth muscle cells. Cell Calcium. 2005;37:25–34. doi: 10.1016/j.ceca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 4.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 6.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglesias R, Spray DC, Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes. 2009;16:131–137. doi: 10.3109/15419061003642618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, Marceau F, Thorin E, Sevigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- 12.Sumi Y, Woehrle T, Chen Y, Yao Y, Li A, Junger WG. Adrenergic receptor activation involves ATP release and feedback through purinergic receptors. Am J Physiol Cell Physiol. 2010;299:C1118–1126. doi: 10.1152/ajpcell.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.