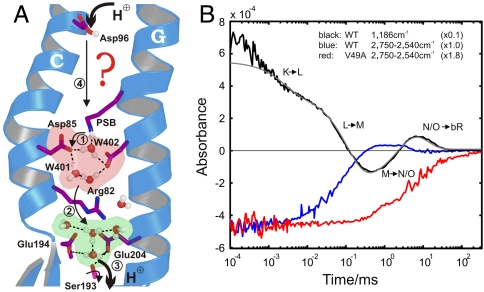
Fig. 1.
Protein-bound water molecules and their IR absorbance changes in bR. (A) Under illumination, bR performs a light-driven proton-pumping cycle including the intermediates J, K, L, M, N, and O (in order of appearance). Following the appearance of M at the end of the first cycle phase, PSB protonates Asp85 (11) (step 1), which cleaves its salt bridge to Arg82. The released Arg82 turns toward Glu194 and Glu204 and pushes the delocalized proton of a protonated water cluster (5) toward these residues (step 2). Transient protonation of Glu194/Glu204 opens the proton diode gate at Ser193, releasing a proton to the extracellular medium (step 3) (9). To reestablish the initial state, SB is reprotonated by Asp96 in N (step 4) (11, 12), which receives a proton from the cytosol in O (12), simultaneous to the isomerization back to all-trans retinal. In the last step Asp85 reprotonates the water cluster at the release site (5, 9). (B) Time-resolved absorbance changes of strong H-bonded water molecules between 2,750 and 2,540 cm-1 for the WT protein are depicted in blue. For comparison, the C14-C15 retinal stretching vibration (13) absorbance change at 1,186 cm-1 is shown in black, reflecting the (unresolved) appearance of the 13-cis isomer, the deprotonation of SB in the L-M transition (step 1), and reprotonation in the M-N transition (step 4). Finally, isomerization back to all-trans retinal takes place in the N-O transition and the absorbance change relaxes to the base line. The N-O transition is not resolved individually due to significant back reactions among the O, N, and M intermediates. The infrared absorbance between 2,750 and 2,540 cm1 in the V49A mutant is depicted in red, which reflects only the change of Wat402. The additional positive signal in M for WT kinetics (blue) reflects the appearance of strong H-bonded water.