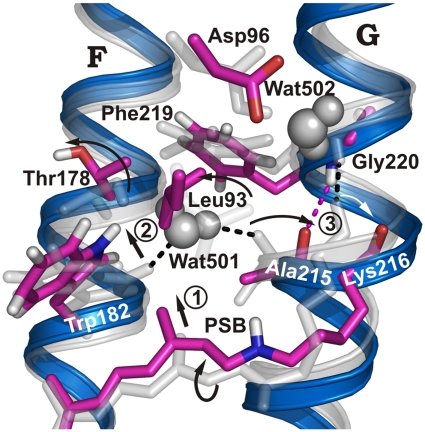
Fig. 2.
Conformational changes in N compared to bR ground state. bR ground-state structure (gray) and simulated N structure (blue/purple). The retinal moves “upward” and the deprotonated SB reorients toward Asp96, causing the twisted conformation of the retinal, especially around the C14-C15 bond, to relax in M (13, 24) (step 1). The movement of the retinal C13-methyl group shifts Trp182 (step 2), which cleaves the H bond to Wat501 and moves helix F by 1 Å. Wat501 is also H-bonded to Ala215 in helix G, where a π-bulge is present in the ground state, resulting in a nonhelical H-bonded Ala215 backbone carbonyl group (1). Retinal relaxation applies a different conformational restraint to the Lys216 backbone. As a result, helical hydrogen bonding changes from Lys216/Gly220 to Ala215/Gly220, and an outward-oriented carbonyl group is found for Lys216 rather than Ala215 (step 3; Fig. S4).