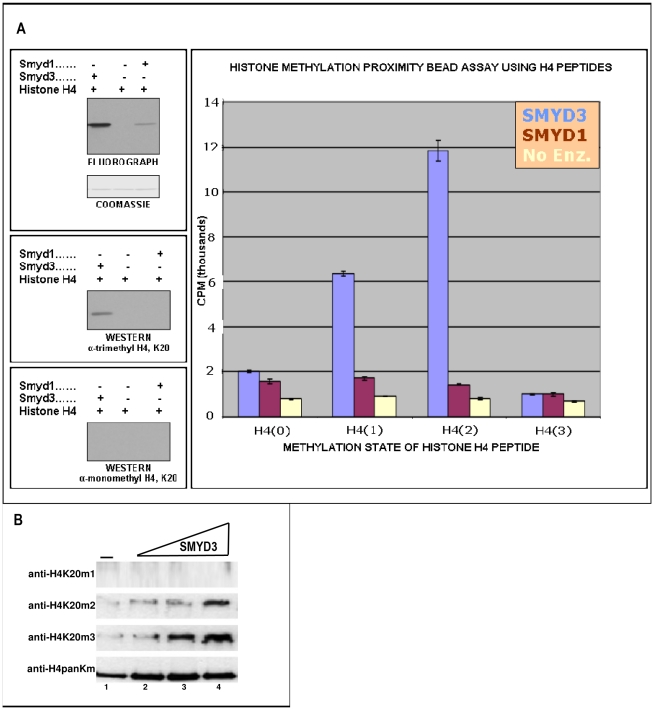
Figure 3. SMYD3 trimethylates H4-K20 preferentially.
(A) SMYD3 trimethylates H4-K20. Right panel: unmethylated [H4(0)], mono-[H4(1)], di-[H4(2)], and, as a negative control, tri-methylated [H4(3)] peptides were employed in an in vitro HMTase proximity bead assay with baculoviral SMYD3 and SMYD1 (negative control). Degree of methylation was measured by scintillation counting in CPM. Left panels: Western analysis using anti-mono- and trimethyl-specific antibodies (Upstate) confirm in vitro specificity of SMYD3 for H4-K20me3. (B) SMYD3 preferentially trimethylates H4-K20 in reconstituted chromatin. Recombinant oocyte nucelosomes were assembled into chromatin, followed by in vitro HMTase assays and SDS-PAGE resolution of reaction products. SMYD3 inputs were increased from 0.5 µg to 2.4 µg, (triangle above lanes), and western analyses were performed with the indicated histone H4 methylation state-specific antibodies (middle panels), with a pan-anti-H4 (lower panel) providing a loading control for chromatin input.