Abstract
Introduction
NS5806 activates the transient outward potassium current (Ito) in canine ventricular cells. We compared the effects of NS5806 on canine atrial versus ventricular tissues and myocytes.
Methods and Results
NS5806 (10 μM) was evaluated in arterially-perfused canine right atrial and right ventricular wedge preparations. In ventricular wedges NS5806 (10 μM) accentuated phase 1 in epicardium (Epi), with little effect in endocardium (Endo), resulting in augmented J-waves on the ECG. In contrast, application of NS5806 (10 μM) to atrial preparations had no effect on phase 1 repolarization but significantly decreased upstroke velocity (dV/dt) and depressed excitability, consistent with sodium channel block. Current and voltage-clamp recordings were made in the absence and presence of NS5806 in (10 μM) enzymatically dissociated atrial and ventricular myocytes. In ventricular myocytes, NS5806 increased Ito magnitude by 80% and 16% in Epi and Endo, respectively (at +40 mV). In atrial myocytes, NS5806 increased peak Ito by 25% and had no effect on the sustained current, IKur. Under control conditions, INa density in atrial myocytes was nearly double that in ventricular myocytes. NS5806 caused a shift in steady-state mid-inactivation (V½) from −73.9±0.27 to −77.3±0.21 mV in ventricular and from −82.6±0.12 to −85.1±0.11 mV in atrial cells, resulting in reduction of INa in both cell types. Expression of mRNA encoding putative INa and Ito channel subunits was evaluated by qPCR.
Conclusion
NS5806 produces a prominent augmentation of Ito with little effect on INa in the ventricles, but a potent inhibition of INa with little augmentation of Ito in atria.
Keywords: Transient outward potassium current, sodium current, atria, ventricle, NS5806
INTRODUCTION
The action potential (AP) in cardiac muscle is an important physiological parameter: 1) it is responsible for electrical conduction, 2) it modulates the refractory period and 3) associated with each action potential is a contraction via the process of excitation-contraction coupling. Marked differences in the shape and duration of the cardiac action potential have been described in different regions of the mammalian heart, reflecting differences in the underlying ionic currents. For example, comparative studies of K+ currents in atrial and ventricular tissue have shown that the inward rectifier K+ current is large in ventricle versus atria1,2 and the ultra rapid delayed rectifier K current, IKur, is large in atria but small3 or virtually absent in the ventricle4. These differences in the complement of K+ currents between the 2 tissue types have been ascribed to variations in the molecular subunits5–7. Marked differences in the kinetics and properties of Na+ current have also been described in the 2 tissue types8–10. Na+ current in atrial tissue has a larger current density and more negative steady-state inactivation compared to ventricular tissue; these differences are thought to contribute to the atrial-selective anti-arrhythmic effects of many compounds.
In atrial and ventricular tissue from dog, the action potential exhibits a large, rapid phase 1 repolarization, suggesting the presence of a Ca2+-independent transient outward K+ current (Ito). Ito is thought to be mediated by Kv4.311,12 together with K+ Channel Interacting Protein (KChIP2)13. The expression of KChIP2 is high in the epicardium (Epi) and midmyocardium (Mid), and low in the endocardium (Endo) which is believed to account for the transmural gradient of Ito14,15 but other proteins, such as the dipeptidyl aminopeptidase-like protein (DPP6)15,16 and KCNEs17,18 may also associate with Kv4.3 and affect current. It has been suggested that Kv1.4 contributes to canine ventricular Ito and although Kv1.4 is uniformly expressed across the ventricular wall15,19, it may have a relatively larger contribution to Endo Ito, where KChIP2 expression is lower, resulting in smaller Kv4.3/KChIP2 current compared to the Epi- and Mid. The slow recovery from inactivation of Endo Ito compared to Epi- and Mid15 provide further support for a role of Kv1.4. The molecular composition of the channels contributing to phase 1 repolarization in the canine atria is also debated. Besides Ito, a slower inactivating, ultra rapid delayed rectifier current (IKur) is present. It is believed that Kv1.5 mediates canine IKur similar to human atrial IKur6.
We have recently identified a compound, NS5806 that produces an increase in the size of current carried through Kv4.3/KChIP2 channels expressed in both Xenopus laevis oocytes and CHO-K1 cells. Similarly, this compound activates Ito in canine ventricular Epi- and Mid cells, whereas minimal effects on Endo Ito were observed. In this study, we investigated the effect of NS5806 on canine atrial currents compared to effects on ventricular currents. Application of NS5806 resulted in about a 30% increase in the magnitude of Ito in atria, a 80% increase in Epi Ito, and no significant effect on Endo Ito and. Surprisingly, application of NS5806 caused a marked reduction in the magnitude of Na+ current in atrial cells compared to ventricular cells. Preliminary results have been presented in abstract form20.
METHODS
NS5806
NS5806 was synthesized at NeuroSearch A/S, Ballerup, Denmark by reaction of 6-cyano-2,4-dibromoaniline with sodium azide to form the respective 2,4-dibromo-6-tetrazolylaniline, which was condensed with 3,5-bis-trifluoromethyl-phenylisocyanate to provide the target 1-[2,4-dibromo-6-(1H-tetrazol-5-yl)-phenyl]-3-(3,5-bis-trifluoromethyl-phenyl)-urea (NS5806). NS5806 was dissolved in DMSO (20 mM stock).
Experimental animals
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85–23, Revised 1996) and was approved by the Animal Care and Use Committee of the Masonic Medical Research Laboratory. Adult mongrel dogs of either sex were used to perform all experimental series. The animals were anticoagulated with heparin and anesthetized with pentobarbital (35mg/kg, i.v.). Their hearts were rapidly removed and placed in cold (4°C) cardioplegic solution (in mM): NaCl 129, KCl 12, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, glucose 5.5.
Isolated ventricular and atrial preparations
Experiments were performed using isolated arterially perfused canine right atrial (RA) preparations or right ventricular (RV) wedge preparations. The RV wedges (2 × 1.5 × 1 cm) were excised from the base of the right ventricular free wall. A descending branch of the right coronary artery (RCA) was cannulated to deliver the perfusate. The isolated atrial preparations were perfused through the ostium of the RCA. During the cannulation procedure, the preparations were perfused with cardioplegic solution (4° C Tyrode’s containing 12 mM KCl) and arterial branches were ligated using silk ligatures. Subsequently, the preparations were placed in a tissue bath and perfused with Tyrode’s solution of the following composition (mM): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, glucose 5.5, bubbled with 95% O 2 and 5% CO2 (37±0.5°C). The perfusate was delivered at a constant flow. Pacing stimuli was delivered to the Endo surface at 2x the diastolic excitability at a basic cycle length (BCL) of 1 s. Control recordings were obtained 1–1.5 hours after mounting; the compounds were applied for 30 min before recordings. A pseudo-ECG was recorded with 2 electrodes consisting of Ag/AgCl half-cells placed 1.0 to 1.2 cm from 2 opposite sides of the atrial preparation. In the RV ventricular wedge, the electrodes were position facing the Endo and Epi surfaces (Epi, positive pole). Intracellular recordings were obtained using floating glass microelectrodes (Endo, approx. 2–3 mm from the endocardial surface, Epi, approx 0–3 mm from the Epi surface, atria, from the pectinate muscle (PM) and the crista terminalis (CT)). ECG and AP signals were amplified and digitized and analyzed using Spike 2 for Windows (Cambridge Electronic Design [CED], Cambridge, UK).
Isolation of adult canine cardiomyocytes
Myocytes from the Epi and Endo region were prepared from canine ventricles or atria. Left ventricular or right atrial preparations were dissected as described above. The preparation was initially perfused with nominally Ca2+-free solution (mM): NaCl 129, KCl 5.4, MgSO4 2.0, NaH2PO4 0.9, glucose 5.5, NaHCO3 20, bubbled with 95% O2/5% CO2 containing 0.1% BSA for a period of about 5 minutes. The preparation was then subjected to enzyme digestion with the nominally Ca2+-free solution supplemented with 0.5 mg/ml collagenase (Type II, Worthington), 0.1 mg/ml protease (Type XIV, Sigma) and 1 mg/ml BSA for 8–12 minutes. For isolation of ventricular cells, thin slices of tissue from the Epi and Endo were shaved from the wedge using a dermatome. For atrial cells, the pectinate muscle was isolated. The tissue was then placed in separate beakers minced and incubated in fresh buffer containing 0.5 mg/ml collagenase, 1 mg/ml BSA and agitated. The supernatant was filtered, centrifuged at 200 rpm for 2 minutes and the myocyte containing pellet was stored in 0.5 mM Ca2+ HEPES buffer at room temperature.
Voltage Clamp Recordings
Cells were placed in a temperature controlled chamber (PDMI-2, Medical Systems Corp.) mounted on the stage of an inverted microscope (Nikon TE300). Voltage-clamp and current-clamp recordings were made using a MultiClamp 700A amplifier and MultiClamp Commander (Axon Instruments). Patch pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, PA). The pipettes were pulled using a gravity puller (Model PP-830, Narishige, Tokyo, Japan) and the pipette resistance ranged from 1–3 MΩ. Initially the cardiomyocytes were superfused with a HEPES buffer of the following composition (mM): NaCl 126, KCl 5.4, MgCl2 1.0, CaCl2 2.0, HEPES 10, and glucose 11. pH adjusted to 7.4 with NaOH. The patch pipette solution had the following composition (mM): K-aspartate 90, KCl 30, glucose 5.5, MgCl2 1.0, EGTA 5, MgATP 5, HEPES 5, NaCl 10, pH=7.2 with KOH and APs were recorded in this solution. For Ito recordings the HEPES buffer was supplemented with 300 μM cadmium to block ICa as previously described21. Sodium currents were recorded as previously described22 in a low Na+ extracellular solution (in mM): CaCl2 0.5, Glucose 10, MgCl2 1.5, Choline-Cl 120, NaCl 5, HEPES 10, KCl 4, Na acetate 2.8, CoCl2 1, BaCl2 0.1, pH=7.4. The pipette solution consisted of: MgCl2 1, NaCl 15, KCl 5, CsF 120, HEPES 10, EGTA 10, Na2ATP 4, and the pH was adjusted to 7.2. All experiments were performed at 37° C except for the sodium current recordings which were performed at room temperature. After a whole cell patch was established, cell capacitance was measured by applying −5 mV voltage steps. Electronic compensation of series resistance to 60–70% was applied to minimize voltage errors. All analog signals (cell current and voltage) were acquired at 10–50 kHz, filtered at 4–6 kHz, digitized with a Digidata 1322 converter (Axon Instruments) and stored using pClamp9 software
Quantitative Real Time PCR
mRNA extraction and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously reported15. The pre-designed gene expression assay from Applied Biosystems was, in addition to the previously reported, cf02625015 (SCN5A). The primers and probes targeting SCN1B, SCN2B, SCN3B, and SCN4B were designed and synthesized by Applied Biosystems, following submission of sequences including intron-exon boundaries using Primer Express 3.0 software.
Statistical Analysis
Data are presented as Mean±SEM. Statistical comparisons were made using paired or unpaired Student’s t-test or one way ANOVA followed by Student Newman-Keuls post test for multiple comparison. p < 0.05 was considered statistically significant (*).
RESULTS
As an initial basis of comparison, we tested the effect of NS5806 in arterially perfused right atrial preparations (Figure 1A). Application of 10 μM NS5806 had no significant effect on phase 1 repolarization (from 5.1±2.1% in control to 6.3±3.7% of phase 2 amplitude, n=4). Action potential duration (APD) was virtually unchanged following 10 μM NS5806 (APD90=223±9.7 ms before and 247±13.8 ms after application of NS5806, n=4, p=N.S.) and the effective refractory period was158±11 ms in control and 183±10 ms in presence of NS5806 (n=4, p=N.S.). At 15 μM NS5806, the atrial action potential showed a depressed phase 0 depolarization and in 2 out of 4 preparations, the depressed phase 0 depolarization deteriorated into complete loss of excitability suggesting block of sodium channels. Similar effects were found on action potentials recorded from the crista terminalis (data not shown).
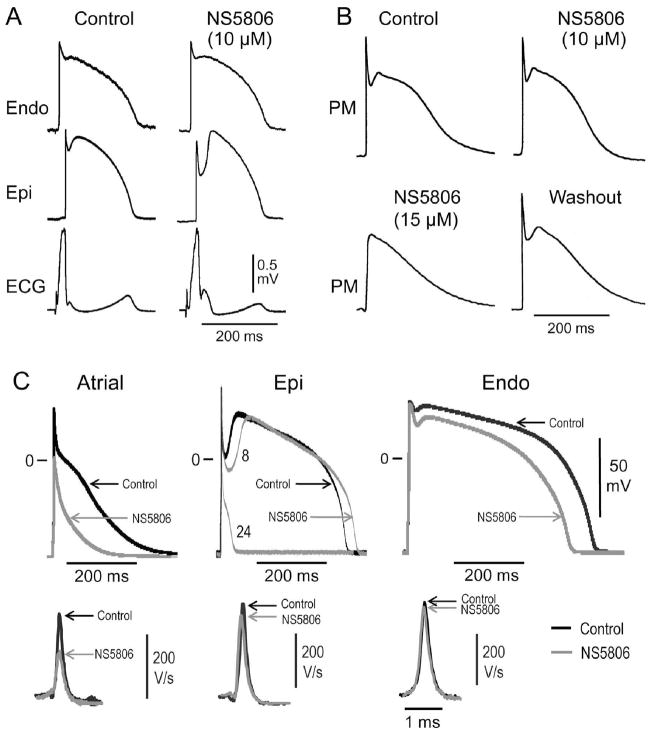
Figure 1.
Effect of NS5806 on arterially perfused atrial and ventricular preparations paced at BCL=1 sec. Panel A) Representative action potentials (AP) recordings from right atrial pectinate muscle (PM) preparation in presence of 0–15 μM NS5806 (n=4). Panel B) Representative AP recordings and corresponding ECG from a right ventricular wedge in absence and presence of 10 μM NS5806 (n=5). Panel C) Representative APs and corresponding dV/dt recorded from isolated canine cardiomyocytes at a BCL of 1 sec. APs recorded from a atrial cell (left panel), LV Epi cell (middle panel), and LV Endo cell (left panel) in absence (black traces) and presence of 10 μM NS5806 (gray traces). The APs recorded from the LV Epi cell were 8 and 24 sec after application of 10 μM NS5806. The results are summarized in Table 1.
In right ventricular preparations, the Epi APs have a prominent phase 1 repolarization and this was further increased by NS5806 (10 μM). In contrast, Endo APs have a small phase 1 repolarization and the APs were unaffected by 10 μM NS5806. The increase in the Epi notch size was accompanied by an accentuation of the J-wave on the ECG (Figure 1B). At 15 μM NS5806, there were minimal effects on the Endo action potential and a prominent enhancement of the spike and dome morphology of the Epi action potential was still present. In addition, there was no significant effect on transmural conduction time, as previously described 22, suggesting the effect of 15 μM NS5806 on ventricular INa is minor.
To further compare the effect of NS5806 on canine atrial and ventricular cells, we characterized the effect of NS5806 on APs recorded from isolated cardiomyocytes. Figure 1C shows APs and corresponding upstroke velocity (dV/dt) in the absence and presence of NS5806. Application of 10 μM NS5806 to isolated right atrial cells (Figure 1C, left panel) dramatically reduced upstroke velocity (dV/dt) by 71±7% (n=7) resulting in a decrease in phase 0 amplitude and in APD90 (Table 1). For Epi cells, application of NS5806 resulted in a dramatic increase in phase 1 repolarization leading to loss of the AP dome, which was accompanied by a significant abbreviation of the APD90. The dV/dt was decreased by 9.8±4.8% (n=5) and phase 0 amplitude unaffected (Figure 1C, middle panel). NS5806 did not have any significant effect on the waveform of Endo APs and dV/dt was reduced by 4.9±2.8% (n=4, p=N.S.) (Figure 1C, right panel). The effect on the different electrophysiological parameters is summarized in Table 1.
Table 1.
Action potentials were recorded from isolated canine atrial or left ventricular Epi or Endo cells at a basic cycle length (BCL) of 1 sec in the absence or presence of 10 μM NS5806. The effects of NS5806 on upstroke velocity (dV/dt), on phase 0 (Ph0), phase 1(Ph1) and phase 2 (Ph2) as well as on action potential duration (ADP) and on resting membrane potential (Erest) of different cell types are summarized.
| Atria | Epi | Endo | |
|---|---|---|---|
| ΔdV/dt | −71±7%, (n=6)*** | −9.8±4.8%, (n=5) * | −4.9±2. 8%, (n=4) * |
| Ph0 Amplitude + NS5806 | 108±4 mV 72±5 mV, (n=6) ** |
124±3 mV 124±3 mV, (n=5), NS |
126±8 mV 125±7, (n=4), NS |
| Notch, Ph1% of Ph2 + NS5806 | - | 28.2±11.1%, (n=5) no Ph2 in 5/5 |
5.5±1.0% 5.1±1.8%, (n=4), NS |
| APD90 + NS5806 | 294±28 ms 187±24 ms, (n=6) *** |
527±46 mS 59±6 mS, (n=5) *** |
535±56 mS 450±28 mS, (n=4), NS |
| Erest + NS5806 | −71.9±0.3 mV −72.6±0.6 mV, (n=6), NS |
−87.1±5 mV −87.4±3 mV, (n=5), NS |
−86.0±6 mV −84.9±6 mV, (n=4), NS |
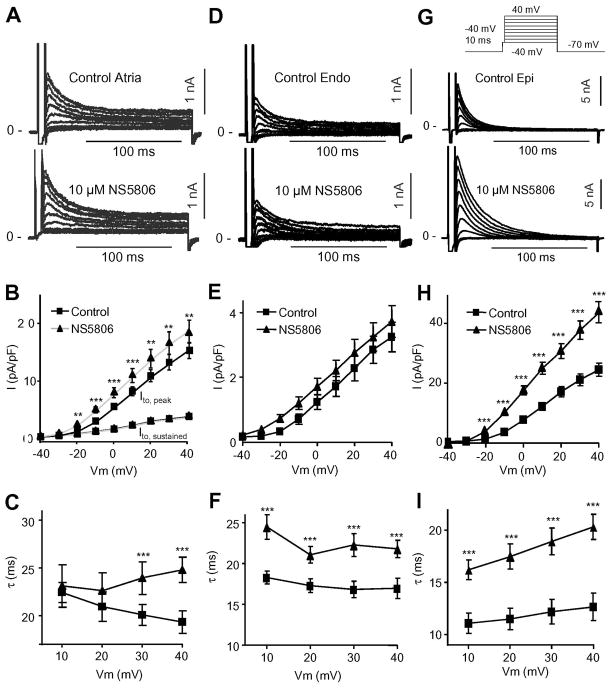
To address the differences in the effect of NS5806 (10 μM) on phase 1 repolarization in atrial and ventricular cells, the density of Ito was measured in isolated canine atrial cells and ventricular Endo and Epi cells. Ito was recorded using whole-cell patch-clamp techniques and Cd2+ (300 μM) was added to the extracellular solution to block ICaL. Following a brief step to −50 mV to discharge sodium channels, voltage steps from −40 to +50 mV were applied and elicited fast activating and rapidly inactivating currents as well as a sustained pedestal current component (Figure 2A). Application of NS5806 significantly increased the peak current density from 15.5±1.6 to 18.9±2.3 pA/pF (at +50 mV, n=14) whereas NS5806 did not affect the sustained current component (from 3.8±0.3 to 3.7±0.3 pA/pF) (Figure 2B). Mono-exponential equations were fit to the current traces and the time constants of decay (τ) following application of NS5806 were significantly slower (from τ=20.0±0.7 to 26.5±1.5 ms at +50 mV) (Figure 2C). For comparison, currents recorded from ventricular Epi and Endo cells using identical experimental conditions are shown in Figure 2D-I.
Figure 2.
Ito in canine atrial and ventricular cells in absence and presence of NS5806 (10 μM). Currents were activated by the voltage clamp protocol (top of figure) and Cd2+ was present to block ICaL. Panel A) Representative Ito traces recorded from canine atrial cells. Panel B) Mean I–V relation for atrial peak Ito as well as for the sustained component Panel C) Ito decay as a function of voltage under control (n=14). Panel D) Representative Ito traces recorded from canine LV Endo cells. Panel E) Mean I–V relation for peak Ito. Panel F) Ito decay as a function of voltage (n=9). Panel G) Representative Ito traces recorded from canine LV Epi cells. Panel H) Mean I–V relation for peak Ito. Panel I) Ito decay as a function of voltage (n=10).
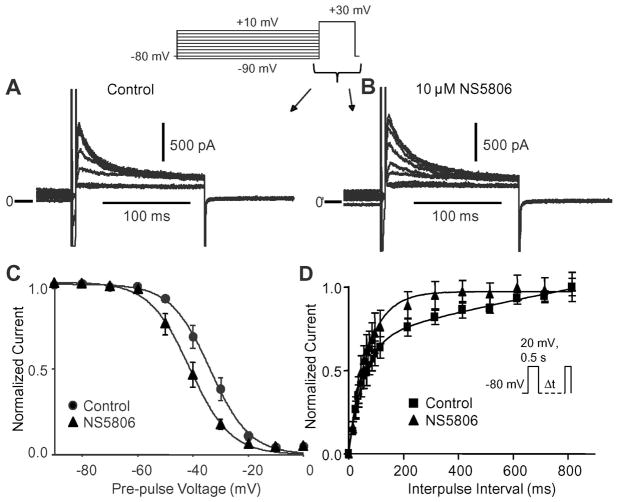
The steady-state inactivation of atrial Ito was evaluated using a prepulse-test pulse voltage-clamp protocol in the presence of Cd2+ and the effect of 10 μM NS5806 was evaluated (Figure 3A and B). The peak current following a 2 s prepulse was normalized to the maximum current and plotted as a function of the prepulse voltage to obtain the availability of the channels. A Boltzmann function was fit to the data (Figure 3C). In the absence of drug, the mid-inactivation voltage was V½=−33.8±0.46 mV and the slope factor k=−7.15±0.40. Application of NS5806 caused a significant negative shift in V½ to −41.1±0.50 mV and k=−7.35±0.42, (n=8). By comparison, the mid-inactivation voltage of Ito was −46.0±1.0 mV, k=−4.75±0.44 for LV Epi cells and −52.9±1.1, k=−8.45±1.34 for LV Endo cells. Application of NS5806 (10 μM) significantly shifted V½ to −52.0±0.9 mV, k=−4.18±0.37 for Epi and −56.3±0.8 mV, k=−7.73±1.07 for Endo cells.
Figure 3.
Voltage dependence of inactivation of atrial Ito. Representative traces recorded under control conditions (Panel A) and after application of 10 μM NS5806 (Panel B) showing voltage dependence of inactivation of Ito. Panel C) The currents were normalized to max-current and plotted as a function of the conditioning pulse and Boltzmann curves were fit to the data. In the absence of drug, the mid-inactivation voltage was V1/2=−33.8±0.46 mV and the slope factor k=−7.15±0.40. In presence of NS5806 V1/2 was −41.1±0.50 mV and k=−7.35±0.42 (n=8). Panel D) Time-dependence of recovery from inactivation of atrial Ito. Currents were elicited by the depicted protocol and normalized recovered currents were plotted as a function of the interpulse interval (n=6).
We next tested if recovery from inactivation of atrial Ito was affected by10 μM NS5806. Currents were elicited by a double pulse protocol, with increasing interpulse intervals. In absence of NS5806, reactivation of Ito showed a fast and a slow phase, with τfast of 55±1.97 ms and a τslow of 2368±327 ms. In the presence of NS5806, the reactivation of atrial Ito conformed to a single exponential equation and was markedly faster with τ=69.3±1.0 ms (n=5). The results are summarized in Figure 3D.
In contrast to Epi cells, atrial cells have a prominent sustained current, IKur. To address the effect of NS5806 on the sustained current, a two-pulse protocol was used to separate IKur and Ito. From a holding of −80 mV, Ito was activated and inactivated by a step to 0 mV for 80 ms. The second pulse was preceded by a 5 ms step to −80 mV and IKur was elicited by a series of voltages ranging from −30 to 40 mV in absence and presence of 10 μM NS580623. Application of NS5806 did not alter peak or sustained IKur (data not shown).
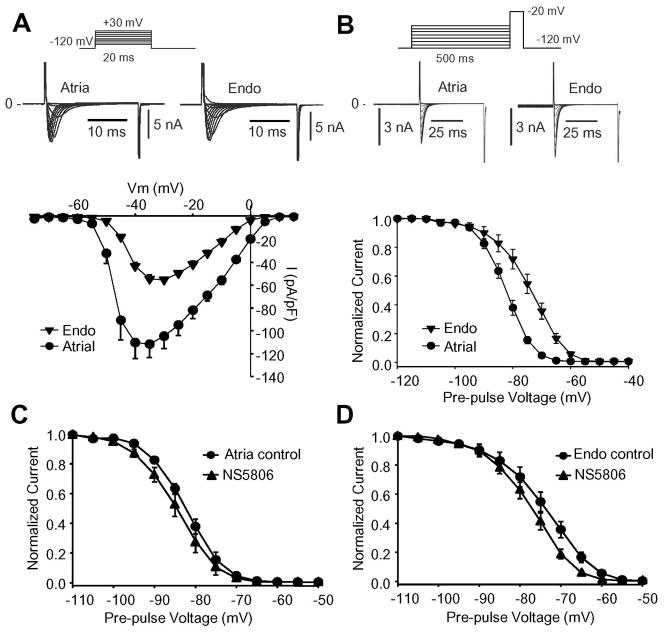
Application of NS5806 to atrial cells resulted in a decrease in dV/dt (Figure 1C and Table 1) suggesting an inhibition of atrial sodium channels. We next recorded INa from cells isolated from canine atrial or ventricular Endo. Endo cells were chosen to minimize the impact of Ito. Representative INa recordings are shown in Figure 4. The current-voltage relationship shows a two-fold higher INa density in atrial cells compared to Endo cells. Steady-state gating parameters were addressed using a standard prepulse-testpulse voltage protocol and mid-inactivation potential (V½) of −82.6±0.12 mV (n=8) for atrial and −73.9±0.27 mV (n=10) for endocardial INa was obtained.
Figure 4.
INa recorded from canine atrial cells or endocardial cells. Cd2+ was present to block ICaL. Panel A) Representative INa traces recorded from atrial (n=8) and Endo cells (n=9) and corresponding mean I–V relation for peak current is shown. Panel B) Representative steady-state inactivation recordings from an atrial or Endo cell. The peak-current following a 500 ms prepulse was normalized to the maximum current and plotted against the conditioning potential to obtain the availability of channels. A Boltzmann equation was fit to the data resulting in a mid-inactivation (V½) of −82.6±0.12 mV (n=8) for atrial and −73.9±0.27 mV (n=9) for Endo I Na. Panel C) Steady-state inactivation for INa recorded from atrial cells in absence and presence of 10 μM NS5806. V½= −82.6±0.1 mV in control and −85.1±0.1 mV in presence of NS5806 (n=8). Panel D) Steady-state inactivation for INa recorded from isolated LV Endo cells in absence and presence of 10 μM NS5806. V½= −73.9±0.27 mV in control and −77.3±0.21 mV in presence of NS5806 (n=9).
As demonstrated in Figure 4C-D, NS5806 induced a highly significant left-shift in mid-inactivation potential V½ for INa. For atrial INa, from V½= −82.6±0.1 mV to −85.1±0.1 mV (n=8) and for Endo INa from V½= −73.9±0.27 mV to −77.3±0.21 mV (n=9). As atrial cells have a more negative V½ compared to Endo cells, this suggest that as cells depolarize, atrial cells would lose excitability at less negative voltages than Endo cells. This tendency would be further enhanced by NS5806 suggesting that the effect of NS5806 on INa could dependent on resting membrane potential.
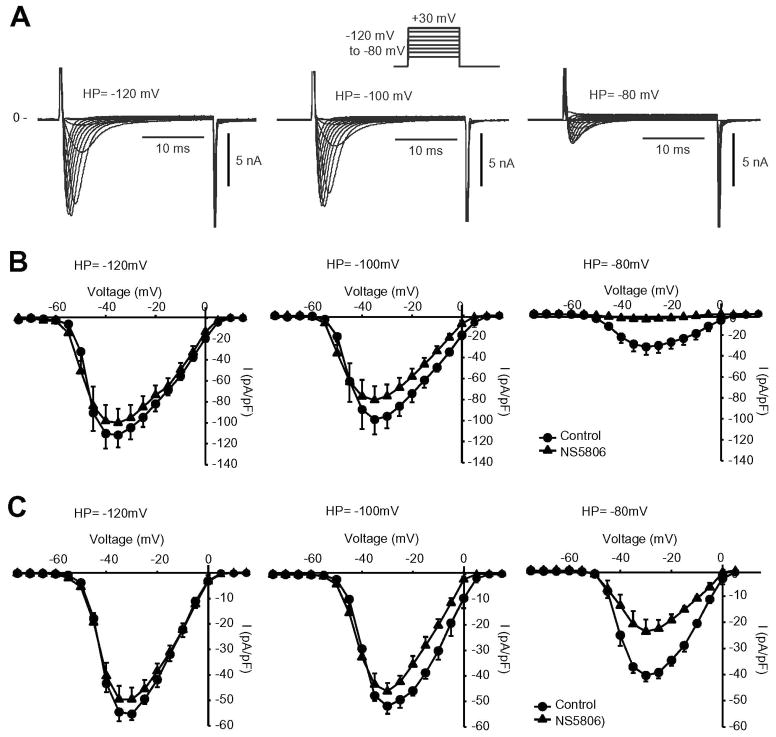
We next tested the effect of 10 μM NS5806 on ventricular and atrial peak INa using holding potentials ranging from −80 to −120 mV as illustrated by the representative atrial INa recordings (Figure 5A). The current-voltage relationships for atrial (Figure 5B) and Endo INa (Figure 5C) recorded at different holding potentials. For both cells types, INa peak currents were smaller when the holding potential was −80 mV versus −120 mV. Application of NS5806 caused a progressive inhibition on I Na and the effect was more pronounced in atrial cells.
Figure 5.
The effect of different holding potentials on atrial and ventricular I Na in absence and presence of 10 μM NS5806. The voltage clamp protocol is shown at the top of the figure and Cd2+ was present to block ICaL. Panel A) Representative INa traces recorded from canine right atrial cells using holding potentials of either −80 mV, −100 mV or −120 mV. Panel B) Mean I–V relation for atrial INa peak current recorded using different holding potentials in absence and presence of 10 μM NS5806 (n=5). Panel C) Mean I–V relation for LV Endo INa peak current recorded using different holding potentials in absence and presence of 10 μM NS5806 (n=5).
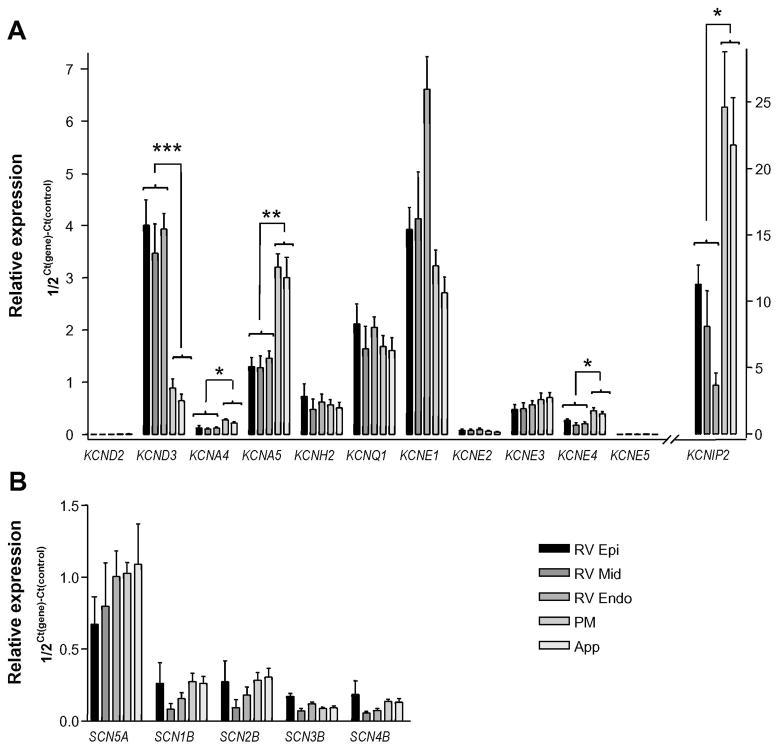
The differential effects of NS5806 on atrial and ventricular Ito and INa suggest different macromolecular ion channel complexes. To determine the expression level of mRNA encoding proteins thought to mediate INa or Ito as well as other K+ currents, we performed qPCR experiments on mRNA isolated from Epi, Mid and Endo as well as from PM and right atrial appendage (App) (Figure 6). We observed a KChIP2 gradient across the right ventricle but no KCND3 gradient (encoding Kv4.3). These observations suggest an Ito gradient is present in the right ventricle, similar to what has been observed in the left ventricle. The relative mRNA expression levels of KCND3, showed an almost 4-fold higher expression in RV compared to RA. In contrast KCNA4 and KCNA5 encoding Kv1.4 and Kv1.5, respectively had a 2 fold higher expression in the atria. KCNH2 encoding Kv11.1 and KCNQ1 encoding Kv7.1 was expressed at similar levels. We also tested the expression of several ancillary subunits and found a high expression of KCNIP2 encoding KChIP2, and a moderate expression of KCNE1, KCNE3 and KCNE4. For KCNIP2, the expression level in the atria was more than 2-fold higher than in ventricle and similar was found for KCNE4. A transmural expression gradient in the RV was also found for KCNE1. We observed no differences in any mRNA levels between PM and App. However, previous studies have shown that the electrophysiology is not uniform throughout the atria8,24 suggesting some differences in ion channel expression throughout the atria. The expression level of the genes encoding proteins suspected to mediate INa was not significantly different between atria and ventricle, nor was any transmural gradient in expression found (Figure 6B)
Figure 6.
qPCR of canine right ventricular (Epi, Mid, and Endo) and right atrial pectinate muscle (PM) and appendage (App) tissue isolated from 5 canine hearts. Taqman based assays (Applied Biosystems) were used to quantify mRNA of interest which was normalized to cyclophilin B expression. Panel A) Expression of K+ channel subunit mRNA. Panel B) Expression of Na+ channel subunit mRNA.
DISCUSSION
In the present work, we compared the effect of NS5806 on Ito and INa in canine atria and ventricle. In canine right ventricular wedges, phase 1 repolarization was increased in Epi while repolarization of Endo was largely unaffected. These changes resulted in an augmented J-wave on the ECG. In contrast, in atrial preparations, phase 1 repolarization was unaffected by NS5806 but excitability was reduced suggesting Na+ channel block.
Effect of NS5806 on Ito
It is believed that Kv4.3 channels comprise the majority of transient outward K+ channels in canine ventricle11,12. Several β-subunits can modify IKv4.3, such as KChIP2 that increases peak IKv4.3 density, slows decay of the current and accelerates recovery from inactivation13. In the left and right ventricle, previous qPCR studies have shown that the Ito gradient across the ventricles is due to a KChIP2 gradient12. This gradient in mRNA was also confirmed at the protein level15. In the present study, qPCR analysis revealed a 3-fold lower expression of Kv4.3 mRNA in atria compared to ventricle; interestingly, the expression of KChIP2 mRNA was 2-fold greater than RV Epi levels. Voltage clamp analysis of Ito showed that Ito was smaller in atria, in agreement with the qPCR data. However, this study raises an important question, namely what is the functional role of excess KChIP2 in canine atria? KChIP2 was initially identified as a β-subunit for Kv4 channels; however, KChIP2 has been found to associate with ICaL25 and INa26 suggesting it may alter the biophysical properties of many ion channels.
Epi Ito was larger than atrial Ito (Figure 2), however atrial cells had a prominent sustained current, IKur that was not observed in Epi cells. NS5806 induced an increase in Ito in both cells types, but the increase was more pronounced for Epi than atrial Ito. The sustained current was unaffected by NS5806. Using qPCR, we found higher expression of Kv1.4 and Kv1.5 mRNA in the atria compared to ventricle. Kv1.4 is thought to mediate a slowly recovering Ito, Ito, slow and Kv1.5 is thought to mediate IKur. For heterologously expressed Kv1.5, 10 μM NS5806 induced a minor block27 consistent with a lack of effect on canine atrial sustained current. For heterologously expressed Kv1.4, application of 10 μM NS5806 resulted in an almost complete block 27. The higher expression of Kv1.4 and Kv1.5 in the atria compared to the ventricle could explain why the relative increase in peak current induced by NS5806 was smaller in the atria than in Epi as NS5806 would enhance a Kv4.3 mediated current component, block a Kv1.4 component of Ito, and leave a Kv1.5 mediated sustained current component unaffected.
The effect on different kinetic parameters of atrial Ito was addressed. Compared to ventricular Ito, atrial Ito had a slower current decay (Figure 2C,F and I). Application of NS5806 further slowed the current decay, similarly to the effects on ventricular I to. In a previous study, we found that NS5806 only slowed current decay of Kv4.3 channels when co-expressed with KChIP222,27, suggesting that at least part of the atrial Ito is mediated by Kv4.3 in complex with KChIP2. The steady-state mid-inactivation potential for atrial Ito was shifted to more negative potentials by NS5806 similar to results seen in ventricular cells15.
Effect of NS5806 on INa
Canine INa is functionally distinct in different areas of the heart. In the ventricles, Epi INa has a significant more negative steady-state mid-inactivation potential compared to Endo28. In agreement with other studies8,10,29,30 we found that atrial INa has a higher current density and a more negative steady-state mid-inactivation potential compared to ventricular Endo INa. The negative steady-state mid-inactivation potential will tend to leave a larger fraction of atrial INa channels in an inactivated state. NS5806 produced a significant shift of steady-state inactivation to more negative potentials for both atrial and ventricular cells; this would be expected to lead to a larger pool of inactivated channels. The more negative mid-inactivation potential and more positive resting membrane potential in the atria (Table 1), can account for the greater depression of INa in atria vs. ventricles. We investigated the level of expression of Nav1.5 subunits in the canine atria and in the three ventricular layers but did not detect significant differences in expression level of mRNA encoding Nav1.5 or Navβ1–4 suggesting mRNA levels measured by qPCR may not reflect protein levels in the membrane, or other proteins and regulatory factors account for the distinct behaviour of the channels.
Effect on action potential waveform
In RV ventricular wedges NS5806 (10 μM) increased phase 1 repolarization in the Epi with no effect on repolarization of Endo as previously described22. In contrast there was no effect on phase 1 repolarization in atrial APs. Surprisingly, NS5806 reduced the AP amplitude, dV/dt and significantly depressed excitability in atrial tissue. NS5806 exerted similar effects in isolated atrial myocytes, whereas the effect on upstroke velocity of APs recoded from isolated ventricular Epi and Endo cells was minor. This disparate effect in atria vs. ventricle is likely due to the greater effect of NS5806 to depress INa for reasons discussed above.
Physiological implications of Ito activation and INa block by NS5806
Many antiarrhythmic agents are effective in terminating atrial fibrillation in both the experimental and clinical setting due to their INa blocking ability. A major limitation of some of these agents is their potential proarrhythmic actions in the ventricle. Previously, it was shown that the antianginal agent, ranolazine was effective at suppressing atrial fibrillation with little effect in the ventricle8. This atrial effectiveness of ranolazine was due to intrinsic differences in sodium channels between atria and ventricle, namely the more negative steady-state inactivation curve8. In the present study, we also found intrinsic differences in sodium channels between atria and ventricle which resulted in greater sodium current inhibition by NS5806 in atria than in ventricle. While the effectiveness of NS5806 in terminating atrial fibrillation was not addressed in the present study, it is clear that NS5806 also exhibits atrial selectiveness in Na+ channel blockade.
Of interest is the mechanism by which NS5806 activates Ito. We have previously shown this compound works through KChIP to cause an increase in the size of Ito15. In the canine ventricle where Kv4.3 levels are equivalent in Epi, Mid and Endo layers, the Ito enhancement was greatest in Epi and Mid cells where KChIP levels are highest. Although atrial tissue has high KChIP levels, Kv4.3 levels were 3 times lower which may explain the modest increase in Ito following application of NS5806 in this tissue type, together with a potential block of atrial IK v1.4 and IKv1.5. In the uncompromised myocardium, application of this compound to ventricular wedges resulted in loss of the epicardial action potential dome and a Brugada-like ECG22. However, in pathophysiological condition such as heart failure, a reduction in Ito due to loss of both Kv4.3 and KChIP levels has been observed31,32. If Kv4.3 and KChIP levels are not severely diminished in heart failure, application of NS5806 may restore Ito and restore the spike and dome morphology.
The influence of action potential waveform and duration on excitation-contraction coupling is well known (for review see Sah et al., 200333). The presence of a spike and dome morphology results in a significant increase in peak ICaL as well as total charge when compared to a cell type where Ito and spike and dome morphology is absent34,35. These observations suggest the spike and dome morphology of the canine Epi action potential is a more efficient releaser of Ca2+ from the SR. In the setting of heart failure, where Ito is known to be reduced, this reduction in Ito would result in reduced Ca2+ influx into the cell via ICaL. Augmentation of Ito in the setting of heart failure may have a beneficial effect on cardiac output by restoring the spike-and-dome morphology resulting in increased Ca2+ influx during the course of an action potential.
In summary, NS5806 produces a prominent augmentation of Ito with little effect on INa in canine ventricles, but a potent inhibition of INa with little augmentation of Ito in canine atria. Molecular analysis of mRNA for atria and ventricle revealed more KChIP2, Kv1.5 and Kv1.4 and less Kv4.3 in atria versus ventricle
Acknowledgments
Financial Support: This work was supported by Eva and Henry Fraenkel foundation [KC], the American Health Assistance Foundation [JMC]; the Danish National Research Foundation [SPO]; the National Institutes of Health [HL47678 to CA] and the Masons of New York State and Florida.
We are grateful to Judy Hefferon, Art Iodice, Robert Goodrow, and Trine Christensen for excellent technical assistance and NeuroSearch A/S for providing NS5806.
Abbreviations
- Ito
transient outward current
- AP
action potential
- Epi
epicardium
- Endo
endocardium
- KChIP2
K Channel Interacting Protein 2
- DPP6
Dipeptidyl aminopeptidase-like protein 6
- PM
pectinate muscle
- CT
crista terminalis
- RV
right ventricle
- LV
left ventricle
- RA
Right Atrial
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design of study; JMC and KC. Collection, analysis and interpretation of data; JMC, KC, NC, EN, TJ, JMD. Drafting the article or revising it critically; JMC, KC, SPO, CA. All authors approved the final version of the manuscript.
Disclosures: SPO is a consultant to NeuroSearch A/S.
References
- 1.Hume JR, Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol (Lond ) 1985;368:525–44. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol (Lond ) 1988;405:123–45. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sridhar A, da Cunha DN, Lacombe VA, Zhou Q, Fox JJ, Hamlin RL, Carnes CA. The plateau outward current in canine ventricle, sensitive to 4-aminopyridine, is a constitutive contributor to ventricular repolarization. Br J Pharmacol. 2007;152(6):870–9. doi: 10.1038/sj.bjp.0707403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedida D, Wible BA, Wang ZG, Fermini B, Faust F, Nattel S, Brown AM. Identity of a novel delayed rectifier current from human heart with a cloned K + channel current. Circ Res. 1993;73:210–6. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- 5.Melnyk P, Zhang L, Shrier A, Nattel S. Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle. Am J Physiol Heart Circ Physiol. 2002;283(3):H1123–H1133. doi: 10.1152/ajpheart.00934.2001. [DOI] [PubMed] [Google Scholar]
- 6.Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93(8):744–51. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- 7.Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94(10):1332–9. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 8.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116(13):1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa T, Koumi S, Sakakibara Y, Singer DH, Jia H, Arentzen CE, Backer CL, Wasserstrom JA. An analysis of lidocaine block of sodium current in isolated human atrial and ventricular myocytes. J Mol Cell Cardiol. 1995;27(2):831–46. doi: 10.1016/0022-2828(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 10.Li GR, Lau CP, Shrier A. Heterogeneity of sodium current in atrial vs epicardial ventricular myocytes of adult guinea pig hearts. J Mol Cell Cardiol. 2002;34(9):1185–94. doi: 10.1006/jmcc.2002.2053. [DOI] [PubMed] [Google Scholar]
- 11.Dixon EJ, Shi W, Wang H-S, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–68. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- 12.Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol. 2003;548(Pt 3):815–22. doi: 10.1113/jphysiol.2002.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deschênes I, DiSilvestre D, Juang GJ, Wu RC, An WF, Tomaselli GF. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac Ito? Circulation. 2002;106(4):423–9. doi: 10.1161/01.cir.0000025417.65658.b6. [DOI] [PubMed] [Google Scholar]
- 14.Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, McKinnon D. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533(Pt 1):119–25. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calloe K, Soltysinska E, Jespersen T, Lundby A, Antzelevitch C, Olesen SP, Cordeiro JM. Differential effects of the transient outward K+ current activator NS5806 in the canine left ventricle. J Mol Cell Cardiol. 2010;48(1):191–200. doi: 10.1016/j.yjmcc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative b-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565(Pt 3):751–6. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delpon E, Cordeiro JM, Nunez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1(3):209–18. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundby A, Olesen SP. KCNE3 is an inhibitory subunit of the Kv4.3 potassium channel. BBRC. 2006;346(3):958–67. doi: 10.1016/j.bbrc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Akar FG, Wu RC, Deschênes I, Armoundas AA, Piacentino V, III, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286(2):H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 20.Calloe K, Chlus N, Nof E, Jespersen T, Olesen SP, Antzelevitch C, Cordeiro JM. Comparison of the effects of the transient outward potassium channel activator NS5806 on canine atrial and ventricular cardiomyocytes. Biophys J. 2010;98:334a. doi: 10.1111/j.1540-8167.2011.02053.x. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumaine R, Cordeiro JM. Comparison of K+ currents in cardiac Purkinje cells isolated from rabbit and dog. J Mol Cell Cardiol. 2007;42(2):378–89. doi: 10.1016/j.yjmcc.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, Antzelevitch C. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81(4):686–94. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue L, Feng J, Li GR, Nattel S. Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. J Physiol. 1996;496 ( Pt 3):647–62. doi: 10.1113/jphysiol.1996.sp021716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng J, Yue L, Wang Z, Nattel S. Ionic mechanisms of regional action potential heterogeneity in the canine right atrium. Circ Res. 1998;83(5):541–51. doi: 10.1161/01.res.83.5.541. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104(12):1382–9. doi: 10.1161/CIRCRESAHA.109.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45(3):336–46. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundby A, Jespersen T, Schmitt N, Grunnet M, Olesen SP, Cordeiro JM, Calloe K. Effect of the I(to) activator NS5806 on cloned K(V)4 channels depends on the accessory protein KChIP2. Br J Pharmacol. 2010;160(8):2028–44. doi: 10.1111/j.1476-5381.2010.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordeiro JM, Mazza M, Goodrow R, Ulahannan N, Antzelevitch C, Di Diego JM. Functionally distinct sodium channels in ventricular epicardial and endocardial cells contribute to a greater sensitivity of the epicardium to electrical depression. Am J Physiol Heart Circ Physiol. 2008;295(1):H154–H162. doi: 10.1152/ajpheart.01327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakakibara Y, Wasserstrom JA, Furukawa T, Jia H, Arentzen CE, Hartz RS, Singer DH. Characterization of the sodium current in single human atrial myocytes. Circ Res. 1992;71:535–46. doi: 10.1161/01.res.71.3.535. [DOI] [PubMed] [Google Scholar]
- 30.Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, Wasserstrom JA. Sodium current in isolated human ventricular myocytes. Am J Physiol. 1993;265(4 Pt 2):H1301–H1309. doi: 10.1152/ajpheart.1993.265.4.H1301. [DOI] [PubMed] [Google Scholar]
- 31.Akar FG, Wu RC, Juang GJ, Tian Y, Burysek M, DiSilvestre D, Xiong W, Armoundas AA, Tomaselli GF. Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;288(6):H2887–H2896. doi: 10.1152/ajpheart.00320.2004. [DOI] [PubMed] [Google Scholar]
- 32.Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561(Pt 3):735–48. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)) J Physiol. 2003;546(Pt 1):5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordeiro JM, Greene L, Heilmann C, Antzelevitch D, Antzelevitch C. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2004;286(4):H1471–H1479. doi: 10.1152/ajpheart.00748.2003. [DOI] [PubMed] [Google Scholar]
- 35.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004;110(20):3168–74. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]