Figure 4.
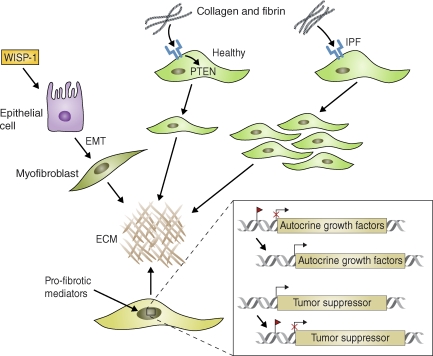
Intrinsic changes in the activation status of epithelial cells and fibroblasts can promote growth factor–independent pulmonary fibrosis. Wnt–β-catenin signaling activated (for example) by WISP-1, is constitutively active in some ATII epithelial cells in IPF patients and in mice with bleomycin-induced pulmonary fibrosis. This signaling triggers EMT and synthesis of ECM components by fibroblasts. In healthy fibroblasts, collagen-mediated stimulation of β1 integrin (blue) up-regulates PTEN activity and inhibits proliferation. IPF fibroblasts, however, display a pathological pattern of β1 integrin expression and signaling that can lead to decreased PTEN expression, aberrant activation of PI3 kinase, and excessive proliferation. Profibrotic mediators also promote epigenetic changes in fibroblasts that contribute to the pathogenesis of fibrosis. For example, the promoter regions of various genes encoding autocrine growth and/or differentiation factors can be demethylated, leading to their sustained and heritable activation. In addition, tumor suppressor genes can become methylated (red flag) and therefore inactivated, leading to the sustained activation of oncogenes that promote growth factor–independent proliferation of fibroblasts. miRNAs (e.g., miR-21) may operate in a similar fashion by blocking the translation or promoting the degradation of tumor suppressor genes in fibroblasts.