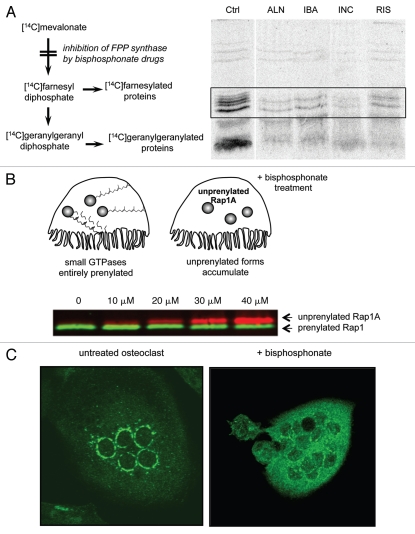
Figure 2.
Bisphosphonate drugs inhibit the prenylation of small GTPases. (A) Inhibition of protein prenylation by nitrogen-containing bisphosphonates can be demonstrated in vitro by culturing cells with [14C]mevalonate, which becomes incorporated into 14C-labeled, prenylated proteins. Radiolabelled, prenylated proteins (21–26 kD, boxed region) can then be detected by autoradiography following electrophoretic separation. Treatment with the bisphosphonate drugs alendronate (ALN), ibandronate (IBA), incadronate (INC) and risedronate (RIS) clearly inhibits prenylation compared with control (Ctrl) cells. Reproduced from Luckman et al.14 with permission of the American Society for Bone and Mineral Research. (B) Inhibition of protein prenylation by bisphosphonate drugs results in the accumulation of the unprenylated form of small GTPases in osteoclasts. The accumulation of unprenylated Rap1A (red) can be determined by protein gel blotting, for example after treatment of cultured cells with ≥10 µM zoledronate (image kindly provided by Gemma Shay). The unprenylated form (red) is of higher molecular mass than the prenylated form (green) due to lack of cleavage of the terminal tripepetide. (C) Inhibition of protein prenylation by bisphosphonate drugs alters the subcellular distribution of small GTPases such as Rab6. Multinucleated osteoclasts were immunostained for Rab6, which localizes to the perinuclear golgi in the untreated osteoclast (left) but has a cytosolic distribution in the osteoclast treated for 48 h with the bisphosphonate risedronate (right).