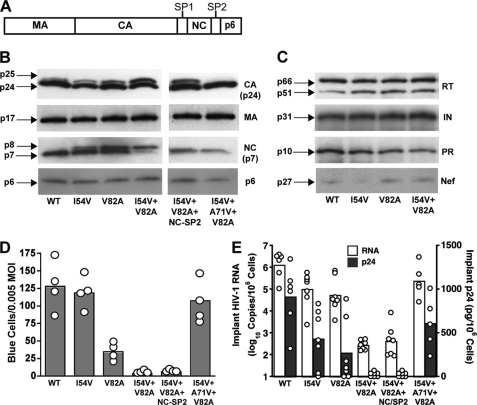
FIGURE 1.
Impaired replication of RTV-resistant HIV results from uncleaved CA-SP1. A, schematic of the protein domain organization in the HIV Gag polyprotein precursor. Proteolytic cleavage of the Gag polyprotein during HIV maturation results in the MA (p17), CA (p24), NC (p7), and p6 proteins. B, Western blot analysis of Gag cleavage products from purified WT HIV and mutant virus particles carrying the indicated RTV resistance mutations in the protease. The right panel shows PR mutant virus particles including compensatory substitutions in the NC-SP2 cleavage junction and the A71V mutation in HIV protease. Insufficient cleavage of Gag is indicated by the presence of p25 (CA-SP1) and p8 (NC-SP2) protein bands. The Western blot is representative of three independent purified virus preparations. C, Western blot analysis of Pol polyprotein and Nef precursor protein cleavage products from wild-type and RTV-resistant HIV particles. Complete cleavage of the Pol polyprotein is indicated by mature RT, IN, and PR proteins. Molecular mass of mature viral proteins is indicated on the left, and the panel is representative of three independent purified virus preparations. D, triplicate wells of P4-Magi cells were inoculated with each virus at an m.o.i. of 0.005 and stained for β-galactosidase activity after 48 h. The columns represent mean number of infected blue cells from four (open circles) independent experiments. E, SCID-hu Thy/Liv mice were inoculated with 1,000 TCID50 of each virus, and Thy/Liv implants were collected 21 days after inoculation. Viral replication was assessed by branched DNA assay for HIV RNA and by ELISA for p24. The columns represent means, and open circles represent individual animals from the same cohort.