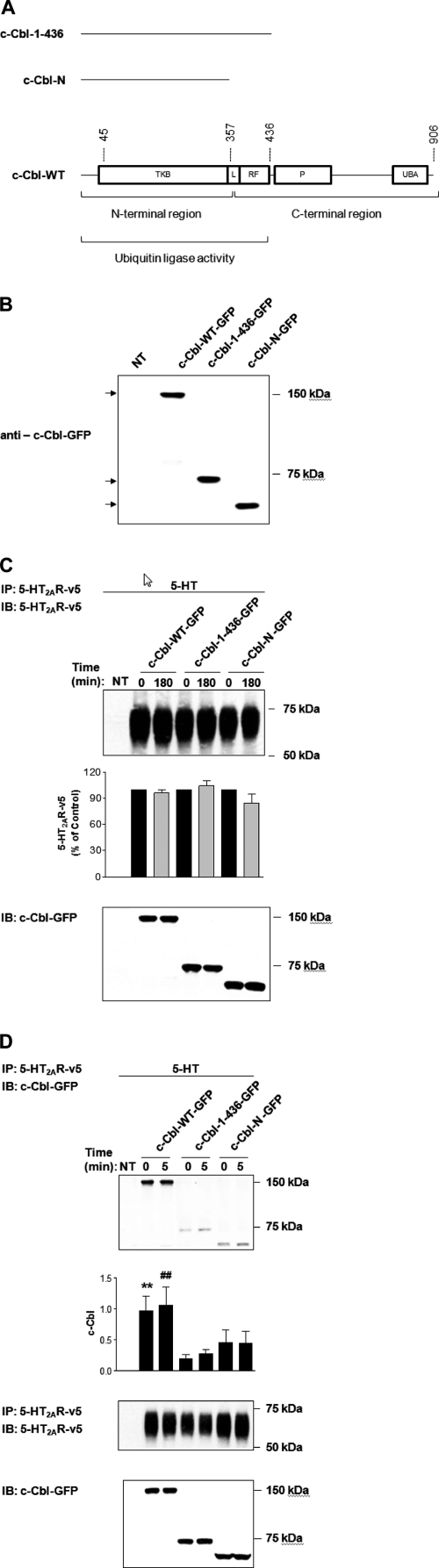
FIGURE 5.
Differential association of c-Cbl mutants with 5-HT2AR. A, schematic representation of full-length c-Cbl (WT) and its two C-terminal truncated mutants (c-Cbl-(1–436) and c-Cbl-N); TKB, tyrosine kinase-binding domain; L, linker region; RF, RING finger domain; P, proline-rich region; UBA, ubiquitin-associated domain. HEK293 cells, which had been either NT or transiently transfected with c-Cbl-WT-GFP, c-Cbl-(1–436)-GFP, or c-Cbl-N-GFP (B) and 5-HT2AR-v5 for 24 h (C and D), were (B) immunoblotted with anti-c-Cbl-GFP antibody, or (C and D) treated with 1 μm 5-HT for (C) 0 or 180 min, or (D) 0 or 5 min, subjected to immunoprecipitation (IP) with an antibody to the 5-HT2AR-v5, followed by immunoblotting (IB) with antibodies to 5-HT2AR-v5 (C) or c-Cbl-GFP (D). Blots in D were then stripped and reprobed for the total amount of 5-HT2AR-v5. Cell lysates in C and D used for immunoprecipitation were also probed for the total amount of c-Cbl-WT-GFP, c-Cbl-(1–436)-GFP, and c-Cbl-N-GFP to normalize for protein expression. Data shown are representative of three independent experiments. Results are mean ± S.E. (n = 3). ** indicates a p of <0.01 versus c-Cbl-(1–436) “0”; ## indicates a p of <0.01 versus c-Cbl-(1–436) “5.”