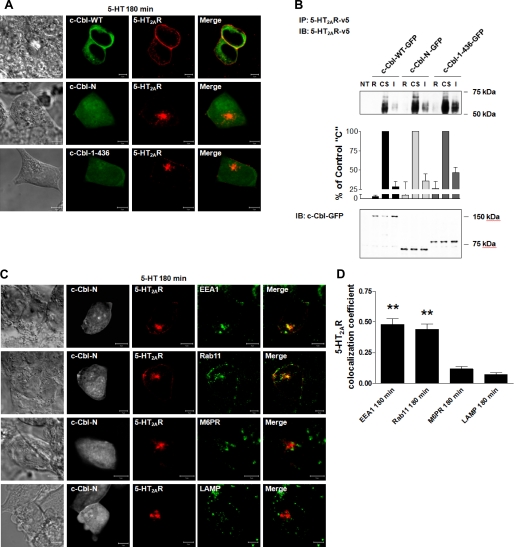
FIGURE 6.
Role of c-Cbl carboxyl terminus in 5-HT2AR recycling. 180 min following a synchronized pulse of 1 μm 5-HT, serum-deprived HEK293 cells, transiently co-transfected with (A) c-Cbl-WT-GFP, or c-Cbl-(1–436)-GFP, or c-Cbl-N-GFP and 5-HT2AR-v5, or (C) c-Cbl-N-GFP and 5-HT2AR-v5 for 24 h, were fixed, stained with anti-v5 antibody (visualized with Alexa Fluor 568-conjugated secondary antibody (red)) and anti-EEA1, -Rab11, -M6PR, or -LAMP antibodies (visualized with Alexa Fluor 633-conjugated secondary antibody (green)), and analyzed by confocal microscopy. Data shown are representative of three independent experiments. Yellow indicates colocalization. The bar is 5 μm. B, following cell surface biotinylation with a cleavable biotin, serum-deprived HEK293 cells, which had been either NT or transiently transfected with c-Cbl-WT-GFP, c-Cbl-(1–436)-GFP, or c-Cbl-N-GFP and 5-HT2AR-v5 for 24 h, were incubated on ice with 1 μm 5-HT for 60 min, washed free of unbound ligand, and either collected following stripping of cell surface biotin (biotin removed, R), or collected without biotin stripping (cell surface receptors, CS), or collected following exposure to prewarmed ligand-free medium at 37 °C for 180 min and cell surface biotin stripping (intracellular receptors, I). Cell lysates were then subjected to immunoprecipitation (IP) with an antibody to 5-HT2AR-v5, followed by immunoblotting (IB) with an antibody to 5-HT2AR-v5. Cell lysates used for immunoprecipitation were also probed for the total amount of c-Cbl-WT-GFP, c-Cbl-(1–436)-GFP, and c-Cbl-N-GFP. Data shown are representative of three independent experiments. Results are mean ± S.E. (n = 3). D, the colocalizations between the 5-HT2AR-v5 and the respective markers observed in panels C were quantified, as explained in the legend of Fig. 2. ** indicates a p of <0.001 versus M6PR and LAMP.