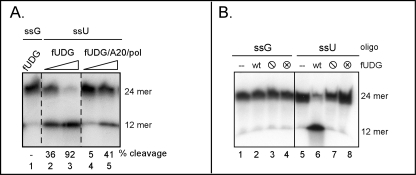
FIGURE 4.
Characterization of the glycosylase activity of the vaccinia virus UDG. A, UDG within the polymerase holoenzyme is enzymatically active. A 5′-radiolabeled 24-mer oligo containing a single uracil residue at position 12 (ssU) was incubated with increasing concentrations of either fUDG (lanes 2 and 3) or a preparation of trimeric holoenzyme (fUDG-A20-Pol) that was free of any uncomplexed fUDG (lanes 4 and 5). A control oligonucleotide containing a G residue (ssG) at position 12 was also incubated with fUDG (lane 1). Reactions were treated with piperidine before separation by denaturing gel electrophoresis and visualization by autoradiography. The migration of the radiolabeled substrate (24-mer) and resultant UDG-dependent cleavage product (12-mer) are noted. B, characterization of UDG variants is shown. The control or uracil-containing oligonucleotide were left untreated (−) (lanes 1 and 5) or incubated with equivalent amounts of WT fUDG (lanes 2 and 6), fUDG-⦶ (lacks glycosylase activity) (lanes 3 and 7), or fUDG-⊗ (lacks uracil recognition) (lanes 4 and 8). Reaction products were treated with piperidine and separated on a denaturing gel.