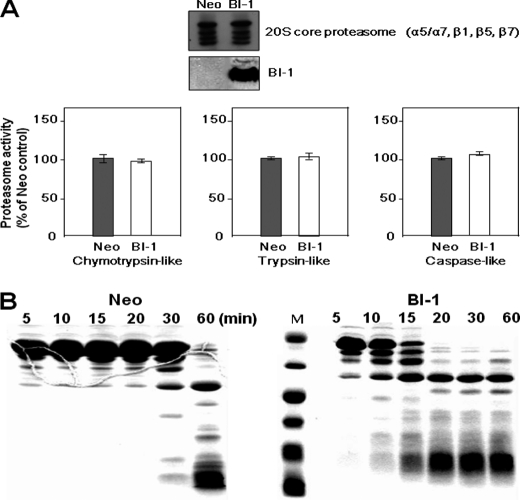
FIGURE 2.
Lysosomal, but not proteasomal, degradation is activated in BI-1-overexpressing cells. A, in both Neo and BI-1 cells, the expressions of proteasome proteins and BI-1 were measured by Western blotting using an antibody against the 20 S core proteasome and HA. Proteasomal activities were calculated by the measurement of peptidolytic activities (chymotrypsin-like, trypsin-like, and caspase-like peptidases). Fluorogenic substrates were incubated with cell extracts for 30 min in the presence of 2 mm ATP, and proteasomal activity was determined by the measurement of fluorescence. Each value is expressed as the mean ± S.E. of three independent experiments. B, after isolation of the lysosomal fraction from Neo and BI-1 cells, 60 μg of BSA in liposome was mixed with 30 μg of lysosomal proteins, and fusion was performed by the addition of 1 mm CaCl2 during the indicated time periods. The collected protein fractions were analyzed by 12.5% SDS-PAGE for evaluation of BSA degradation, as described under “Experimental Procedures.” M, molecular marker.