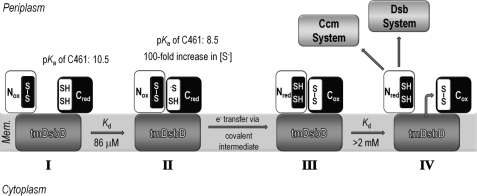
FIGURE 9.
Schematic representation of the reaction cycle involving nDsbD and cDsbD. I, Cys461, the attacking cysteine residue in cDsbD, has an elevated pKa value of 10.5 when cDsbDred is distant from nDsbD (25). This makes Cys461 a poor nucleophile because essentially only the thiol species will be present and protects cDsbDred from nonspecific oxidation by other periplasmic proteins. II, the relatively high affinity of nDsbDox for cDsbDred (Kd = 86 μm) ensures formation of the nDsbDox·cDsbDred complex at the high effective concentration found in intact DsbD. In close proximity to nDsbD, the pKa value of Cys461 in cDsbDred is lowered by as much as 2 pH units (26). This increases the concentration of the reactive thiolate species (S−), making Cys461 a better nucleophile. Cys461 will attack the disulfide bond of nDsbDox, allowing electron transfer to proceed via a mixed-disulfide covalent intermediate. III, the lower affinity of the two protein partners in the nDsbDred·cDsbDox complex (Kd > 2 mm) ensures dissociation following electron transfer. IV, nDsbDred is available for reduction of its periplasmic partners CcmG and DsbC, whereas cDsbDox is available for reduction by tmDsbDred.