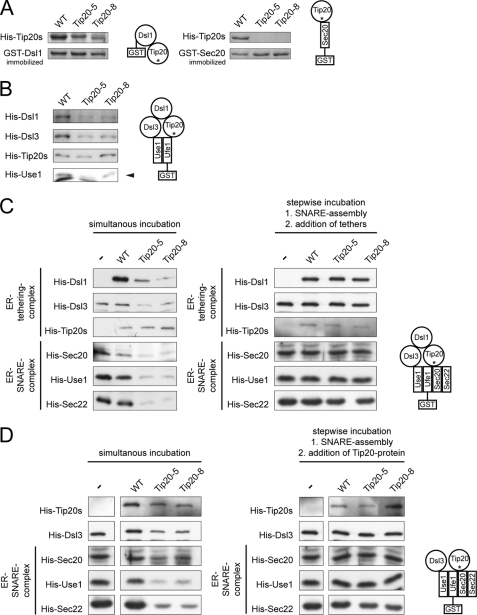
FIGURE 4.
In vitro assembly of Dsl1 tethering complexes and ER trans-SNARE complexes is affected by Tip20p mutants. A, Tip20p mutants have a lower affinity for Sec20 and Dsl1p than wild-type Tip20p. A GST pulldown was performed with GST-Ds1p or GST-Sec20p and Tip20p variants. B, Dsl1p binding to Tip20-5p or Tip20-8p is drastically decreased. To reconstitute the Dsl1 tethering complex in vitro, pulldown assays were performed as indicated in the graphical representation and analyzed by immunoblotting. Note that Dsl3p and Use1p were added as a complex. Dsl1p and Dsl3p were detected with protein specific antibodies, whereas an anti-His antibody was used to detect Tip20p and Use1p. The arrowhead in the Use1p blot points to the upper band, which is the specific one. C, Tip20p is required for SNARE complex assembly. ER SNARE complexes were reconstituted either in the presence or absence of Dsl1 complex members. Mutant Tip20p proteins strongly reduced the amount of SNARE complexes formed. No effect was observed when the Dsl1 complex members were added after SNARE assembly had occurred. D, Dsl1p does not influence SNARE complex assembly. In vitro pulldown assay were performed as in C, but Dsl1p was omitted. The asterisk under Tip20 in the drawings is meant to illustrate the usage of wild type and mutant versions in the pulldowns.