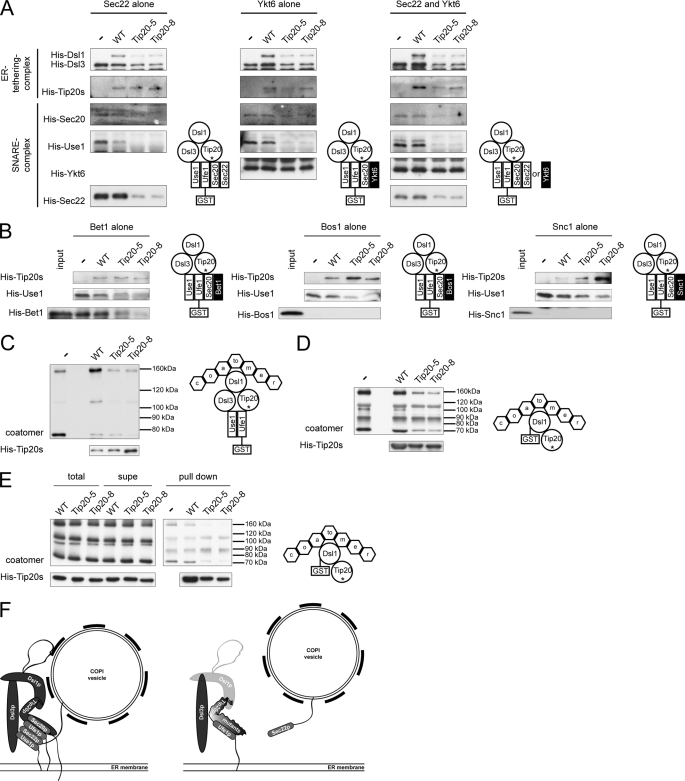
FIGURE 5.
The assembly of ER SNARE complexes in tip20 mutants is not rescued by alternative v-SNAREs and coatomer binding is affected in the presence of Tip20-5p or Tip20-8p. A, Ykt6p binds to GST-Ufe1p but does not promote SNARE complex assembly. Pulldown assays were performed as indicated in the graphical representation. B, Bet1p, Bos1p, and Snc1p do not improve SNARE complex assembly in the presence of the Tip20p mutants. Pulldown assays were performed as indicated in the graphical representation. C, coatomer binding to mutant Dsl1 complexes is reduced in vitro. To assess whether the coatomer binding function of the Dsl1 complex is affected by the Tip20p mutants, Dsl1 complexes were assembled, and coatomer was added either at the same time or only after Dsl1 complex preassembly. D, Tip20-5 and Tip20-8 negatively regulate the interaction between coatomer and Dsl1p. Tip20p variants and coatomer were incubated with GST-Dsl1p and the binding of the proteins to GST-Dsl1p was determined by immunoblot. E, GST-Dsl1p and Tip20p do not compete for the same binding site on coatomer. Preincubation of Tip20p proteins with coatomer did not change the binding behavior. A pulldown experiment was performed as described in D except that coatomer and the Tip20p proteins were preincubated at 4 °C. To eliminate possible aggregation, the reaction mixture was spun for 15 min at 20,000 × g at 4 °C, and the supernatant was used for the binding reaction to GST-Dsl1p. F, scheme describing the results. For an explanation, see text.