FIGURE 3.
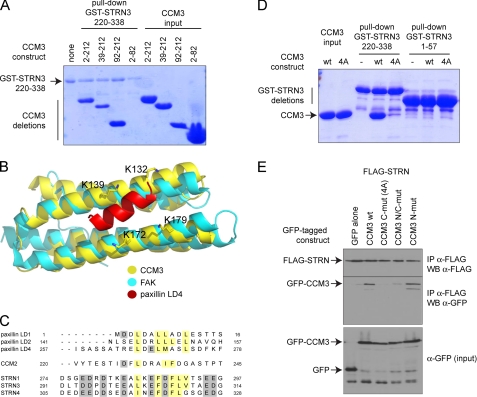
CCM3 interacts with STRN3 via its FAT domain. A. STRN3 interacts with the C-terminal portion of CCM3. A GST pulldown assay was performed with GST-STRN3 220–338 and bacterially expressed and purified CCM3 deletions to map the region on CCM3 responsible for binding to GST-STRN3. Deletion of the first 92 amino acids of CCM3 did not affect the interaction, and a region encompassing amino acids 2–82 was unable to associate to GST-STRN3(220–338). B, structural modeling of the CCM3 FAT domain with a peptide derived from paxillin. The CCM3 crystal structure (30) revealed that the region that we have mapped as interacting with STRN3 also folds as a focal adhesion targeting domain (yellow) similar to focal adhesion kinase (FAK, cyan). Interaction with peptides derived from paxillin (shown in red) are mediated via four lysine residues (highlighted). C, alignment of the peptide derived from STRN3 (and corresponding peptides in the STRN and STRN4 paralogs) with the CCM3 binding regions of CCM2 and paxillin suggests a common mode of association. D, mutation to alanines of the four conserved lysines (Lys-132, Lys-139, Lys-172, and Lys-179) in CCM3 C-mut (4A) abrogates interaction with GST-STRN3(220–338) in vitro. GST pulldown assays were performed with GST-STRN3(220–338) to monitor binding of wild-type CCM3 or CCM3 C-mut (4A). Only wild-type CCM3 is pulled down by GST-STRN3. GST-STRN3(1–57) was used as a negative control. E, mutation to alanines of the four conserved lysines in CCM3 abrogates the interaction with full-length Strn in HEK293T cells. Co-transfection of FLAG-tagged full-length Strn with GFP-tagged CCM3 constructs WT, C-mut (4A), N-mut, and N/C-mut was performed. Immunoprecipitation of FLAG-Strn was followed by immunoblotting with anti-GFP to detect CCM3 association. CCM3 C-mut, 4A is unable to interact with FLAG-Strn, whereas CCM3 N-mut has no effect on the interaction. A combination of both mutations also prevented the interaction, as expected.