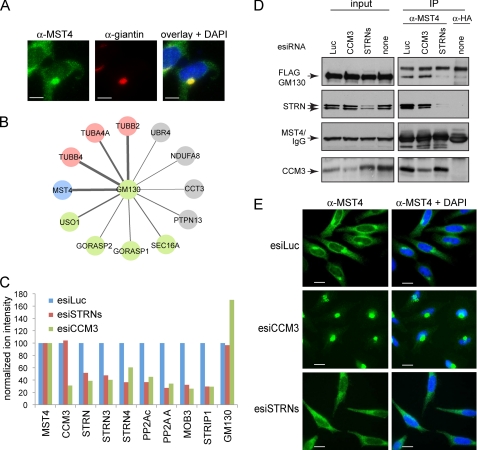
FIGURE 5.
CCM3 and striatins exert opposite effects on MST4 localization. A, co-localization of endogenous MST4 (green) with the Golgi protein giantin (red). In the overlay (right), co-localization to the Golgi is shown in yellow; note that a fraction of MST4 does not localize to the Golgi but is instead detected as green punctae in the cytosol. Scale bar, 7.5 μm. B, GM130 interactors identified by mass spectrometry. AP-MS was performed as described under “Experimental Procedures.” Statistical analysis of the interactions using SAINT was performed; see supplemental Tables 3 and 4 for complete mass spectrometric data. The thickness of the edges is proportional to spectral counts (total number of peptides) for the prey, whereas the color indicates MST4 (blue), known Golgi proteins (green), tubulins (pink), or proteins other than MST4, Golgi proteins, or tubulins (gray). Note that MST4 is a major interaction partner for GM130. C, depletion of CCM3 decreases association of MST4 with STRIPAK but not the interaction with GM130. Stable HEK293 cells expressing FLAG-Mst4 were transfected with the indicated esiRNAs (see Fig. 4C for details). FLAG-Mst4 was immunoprecipitated using anti-FLAG antibodies, and the sample was processed for quantitative mass spectrometry. Relative quantification by mass spectrometry was performed using a TripleTOF 5600 with cells depleted of STRN proteins or CCM3; normalization to Mst4 (bait) levels and to the expression levels in the luciferase samples is shown. See supplemental Table 5 for mass spectrometric results. As shown in Fig. 4C, depletion of CCM3 affects recovery of all STRIPAK components with Mst4; depletion of the striatins affects recovery of all STRIPAK components with the exception of CCM3. By contrast, depletion of CCM3 appeared to increase the GM130 interaction with FLAG-Mst4, indicating that this interaction is not mediated via STRIPAK. D, GM130 interaction with endogenous MST4 is reduced by depletion of striatins in HEK293 cells stably expressing FLAG-GM130. Transfection of esiRNAs was followed by immunoprecipitation of endogenous MST4 and immunoblotting for FLAG-GM130 and STRIPAK proteins. To control for the amount of FLAG-GM130 non-specifically binding to the beads, we performed immunoprecipitation in parallel with an isotype-matched antibody (anti-HA). (There is no HA protein transfected in these cells.) E, esiRNA-mediated depletion of CCM3 in HeLa cells induces near complete localization of MST4 to the Golgi, whereas depletion of striatins prevents Golgi localization. Transfection of indicated esiRNAs was followed by immunofluorescence staining of MST4 and DAPI. Scale bar, 10 μm.