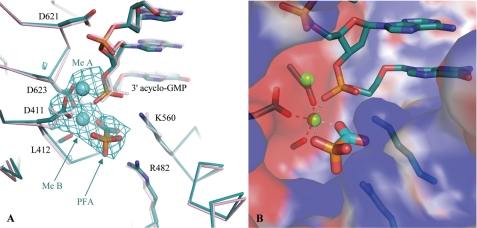
FIGURE 2.
Close-up view of the active site of the chimeric RB69 gp43. A, a superposition of the ternary PFA complex (cyan) and the binary RB69 gp43-DNA complex (pink) is shown. The 2.7 Å Fo − Fo map contoured at 3 σ reveals unbiased density for PFA, as well as metals A and B. Upon binding PFA, the conformation of the strictly conserved catalytic Asp-411 changes. The DNA has failed to translocate, which is indicated by the proximity of the incorporated acyclo-GMP to the catalytic aspartate residues (Asp-411 and Asp-623). B, the surface representation of the active site (blue for positive charges and red for negative charges) illustrates the multiple electrostatic interactions between PFA and the chimeric DNA polymerase.