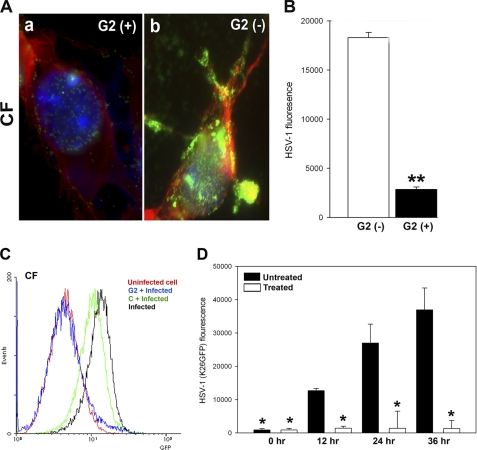
FIGURE 6.
G2 functions by preventing HSV-1 attachment to cells, which results in loss of binding and viral replication. A, GFP-expressing HSV-1(K26GFP) binding to CF in the presence and absence of G2 peptides was examined by fluorescence microscopy. CF were grown in collagen-coated chamber slides and incubated at room temperature for 60 min with G2 (+) or control Cp (G2 (−)) peptide. This was followed by the incubation of the cells in cold with GFP-expressing HSV-1(K26GFP) for 30 min and washing of unbound virioins with PBS. Cells were fixed, stained with phalloidin for F-actin (red) and DAPI for nuclei (blue), and examined by a fluorescence microscope (Leica, SP2). The presence of the virus is shown in green. B, relative virus binding to CF was estimated by fluorescence measurements. Cultured CF were preincubated with G2 and Cp for 60 min before ice-cold incubation with GFP-expressing HSV-1 virus for 30 min. Cells were washed 3 times, and viruses remaining on cell surfaces were assayed for GFP fluorescent intensity using a fluorescence reader (Tecan). C, GFP-expressing HSV-1(K26GFP) intensity as a surrogate for virus binding was quantified in presence G2 or control peptide (abbreviated as C) by flow cytometry. The cell/virus incubation was performed as described above. D, G2 peptides block HSV-1 replication into cultured human CFs. Cultured CF were preincubated with G2 or mock-treated (Cp) before infection with HSV-1(K26GFP) virus for 6 h. Viral replications in CF were quantified 0–36 h post-infection by measuring GFP fluorescent intensity using a fluorescence reader (Tecan). The data shown are the means of triplicate measures and are representative of three independent experiments. Asterisks indicate significant difference from other treatments (p < 0.01, t test); error bars represent S.D.