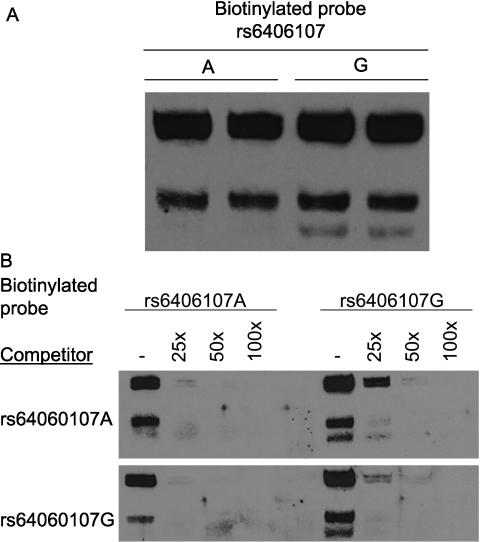
Figure 7.
Single nucleotide polymorphism (rs6406107) in the 5′ untranslated region of Acvr1 diminishes nuclear protein binding capacity. (A) Oligonucleotide probe containing the G-allele, in contrast to the A-allele, formed a distinct fast-migrating complex (lower band). Electrophoretic mobility shift assay (EMSA) of nuclear protein extract prepared from mouse lung epithelial cells (MLE-15) and biotinylated oligonucleotide probes containing the A- or G-allele. (B) Compared with the A-variant, the G-variant is a more effective competitor of protein-biotinylated DNA complexes formation. Competitive EMSA was performed with excess double-strained oligonucleotides that contained either the A- or G-variant. The biotinylated labeled rs6406107 A-variant oligonucleotide probe is readily competed by nonbiotinylated rs6406107 A- or G-variant, whereas the biotinylated labeled rs6406107 G-variant oligonucleotide probe is more avidly bound, in particular the fast migrating complex (right, lane 2).