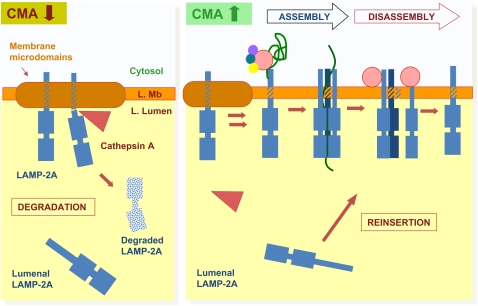
Figure 4.
Local regulation of chaperone-mediated autophagy (CMA) activity in lysosomes. Under conditions of low CMA activity (left), lysosome-associated membrane protein (LAMP)-2A is recruited to lysosomal membrane (L. Mb) microdomains, where it undergoes partial cleavage by cathepsin A, followed by rapid degradation of this truncated product in the lysosomal lumen (L. Lumen). There is a fraction of lysosomal LAMP-2A that resides in the lumen and is not accessible for substrate binding or translocation. When CMA is activated (right), association of LAMP-2A with the membrane microdomains decreases, favoring, instead, binding of substrate proteins to the cytosolic tail of this receptor. Substrate binding promotes multimerization of LAMP-2A to form a translocation complex. Once the substrate is released into the lysosomal lumen, hsc70 present at the lysosomal membrane promotes disassembly of LAMP-2A from the translocation complex. When maximal activation of CMA is required, the pool of LAMP-2A resident in the lysosomal lumen can be mobilized toward the membrane to contribute to substrate binding/uptake.